Design, Synthesis, and Evaluation of Homochiral Peptides Containing Arginine and Histidine as Molecular Transporters
- PMID: 29966296
- PMCID: PMC6100079
- DOI: 10.3390/molecules23071590
Design, Synthesis, and Evaluation of Homochiral Peptides Containing Arginine and Histidine as Molecular Transporters
Abstract
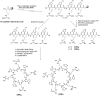
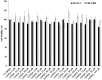
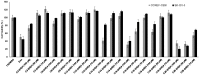
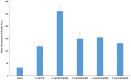
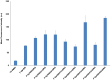
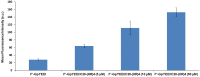
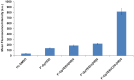
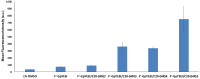
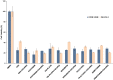
Linear (HR)n and cyclic [HR]n peptides (n = 4,5) containing alternate arginine and histidine residues were synthesized. The peptides showed 0⁻15% cytotoxicity at 5⁻100 µM in human ovarian adenocarcinoma (SK-OV-3) cells while they exhibited 0⁻12% toxicity in human leukemia cancer cell line (CCRF-CEM). Among all peptides, cyclic [HR]₄ peptide was able to improve the delivery of a cell impermeable fluorescence-labeled phosphopeptide by two-fold. Fatty acids of different alkyl chain length were attached at the N-terminal of the linear peptide (HR)₄ to improve the molecular transporter property. Addition of fatty acyl chains was expected to help with the permeation of the peptides through the cell membrane. Thus, we synthesized seven fatty acyl derivatives of the linear (HR)₄ peptide. The peptides were synthesized using Fmoc/tBu solid phase peptide chemistry, purified by reverse-phase high-performance liquid chromatography (RP-HPLC) and characterized by matrix-assisted laser desorption/ionization (MALDI) spectrometry. The fatty acyl peptides containing C₈, C12, C14, and C18 alkyl chain did not show cytotoxicity on SK-OV-3 or CCRF-CEM cell lines up to 50 µM concentration; however, at higher concentration (100 µM), they showed mild cytotoxicity. For example, C16-(HR)₄ was also found to reduce the proliferation of SK-OV-3 cells by 11% at 50 µM and C20-(HR)₄ showed mild toxicity at 10 µM, reducing the proliferation of SK-OV-3 cells by 21%. Increase in the length of alkyl chain showed cytotoxicity to the cell lines. C20-(HR)₄ peptide showed better efficiency in translocation of F′-GpYEEI to SK-OV-3 than the phosphopeptide alone. Further investigation of C20-(HR)₄ peptide efficacy showed that the peptide could deliver doxorubicin and epirubicin into SK-OV-3 and also improved the drug antiproliferative ability. These studies provided insights into understanding the structural requirements for optimal cellular delivery of the fatty acyl-(HR)₄ peptide conjugates.
Keywords: anticancer drugs; cytotoxicity; fatty acyl peptides; histidine; molecular transporters; phosphopeptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

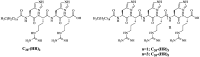
Similar articles
-
Efficient Intracellular Delivery of Cell-Impermeable Cargo Molecules by Peptides Containing Tryptophan and Histidine.Molecules. 2018 Jun 26;23(7):1536. doi: 10.3390/molecules23071536. Molecules. 2018. PMID: 29949881 Free PMC article.
-
Comparative Molecular Transporter Properties of Cyclic Peptides Containing Tryptophan and Arginine Residues Formed through Disulfide Cyclization.Molecules. 2020 Jun 2;25(11):2581. doi: 10.3390/molecules25112581. Molecules. 2020. PMID: 32498339 Free PMC article.
-
Hybrid Cyclic-Linear Cell-Penetrating Peptides Containing Alternative Positively Charged and Hydrophobic Residues as Molecular Transporters.Mol Pharm. 2021 Oct 4;18(10):3909-3919. doi: 10.1021/acs.molpharmaceut.1c00594. Epub 2021 Sep 7. Mol Pharm. 2021. PMID: 34491768
-
The rational design of cell-penetrating peptides for application in delivery systems.Peptides. 2019 Nov;121:170149. doi: 10.1016/j.peptides.2019.170149. Epub 2019 Sep 3. Peptides. 2019. PMID: 31491454 Review.
-
Intramolecular acyl transfer in peptide and protein ligation and synthesis.J Pept Sci. 2015 Mar;21(3):139-47. doi: 10.1002/psc.2749. Epub 2015 Jan 30. J Pept Sci. 2015. PMID: 25641053 Review.
Cited by
-
Analogs of Periplanetasin-4 Exhibit Deteriorated Membrane-Targeted Action.J Microbiol Biotechnol. 2020 Mar 28;30(3):382-390. doi: 10.4014/jmb.1912.12044. J Microbiol Biotechnol. 2020. PMID: 32238758 Free PMC article.
-
Intracellular Delivery of Stabilized Peptide Blocking MTDH-SND1 Interaction for Breast Cancer Suppression.JACS Au. 2023 Dec 8;4(1):139-149. doi: 10.1021/jacsau.3c00573. eCollection 2024 Jan 22. JACS Au. 2023. PMID: 38274259 Free PMC article.
-
Peptide-Based Nanoassemblies in Gene Therapy and Diagnosis: Paving the Way for Clinical Application.Molecules. 2020 Jul 31;25(15):3482. doi: 10.3390/molecules25153482. Molecules. 2020. PMID: 32751865 Free PMC article. Review.
-
Advances in Cytosolic Delivery of Proteins: Approaches, Challenges, and Emerging Technologies.Chem Biodivers. 2025 Jun;22(6):e202401713. doi: 10.1002/cbdv.202401713. Epub 2025 Feb 17. Chem Biodivers. 2025. PMID: 39921680 Free PMC article. Review.
-
Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies.Molecules. 2019 Mar 7;24(5):927. doi: 10.3390/molecules24050927. Molecules. 2019. PMID: 30866424 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

