Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges
- PMID: 29966303
- PMCID: PMC6073924
- DOI: 10.3390/ma11071116
Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges
Abstract
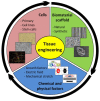
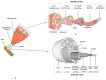
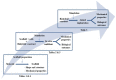
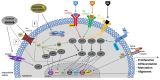
Tissue engineering is a promising approach to repair tendon and muscle when natural healing fails. Biohybrid constructs obtained after cells’ seeding and culture in dedicated scaffolds have indeed been considered as relevant tools for mimicking native tissue, leading to a better integration in vivo. They can also be employed to perform advanced in vitro studies to model the cell differentiation or regeneration processes. In this review, we report and analyze the different solutions proposed in literature, for the reconstruction of tendon, muscle, and the myotendinous junction. They classically rely on the three pillars of tissue engineering, i.e., cells, biomaterials and environment (both chemical and physical stimuli). We have chosen to present biomimetic or bioinspired strategies based on understanding of the native tissue structure/functions/properties of the tissue of interest. For each tissue, we sorted the relevant publications according to an increasing degree of complexity in the materials’ shape or manufacture. We present their biological and mechanical performances, observed in vitro and in vivo when available. Although there is no consensus for a gold standard technique to reconstruct these musculo-skeletal tissues, the reader can find different ways to progress in the field and to understand the recent history in the choice of materials, from collagen to polymer-based matrices.
Keywords: collagen; elastic modulus; electrospinning; sponge; stem cells; stretching.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

