p19-Targeting ILP Protein Blockers of IL-23/Th-17 Pro-Inflammatory Axis Displayed on Engineered Bacteria of Food Origin
- PMID: 29966384
- PMCID: PMC6073689
- DOI: 10.3390/ijms19071933
p19-Targeting ILP Protein Blockers of IL-23/Th-17 Pro-Inflammatory Axis Displayed on Engineered Bacteria of Food Origin
Abstract
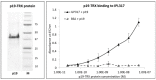
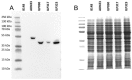
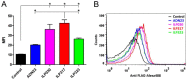
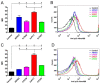
IL-23-mediated Th-17 cell activation and stimulation of IL-17-driven pro-inflammatory axis has been associated with autoimmunity disorders such as Inflammatory Bowel Disease (IBD) or Crohn’s Disease (CD). Recently we developed a unique class of IL-23-specific protein blockers, called ILP binding proteins that inhibit binding of IL-23 to its cognate cell-surface receptor (IL-23R) and exhibit immunosuppressive effect on human primary blood leukocytes ex vivo. In this study, we aimed to generate a recombinant Lactococcus lactis strain which could serve as in vivo producer/secretor of IL-23 protein blockers into the gut. To achieve this goal, we introduced ILP030, ILP317 and ILP323 cDNA sequences into expression plasmid vector containing USP45 secretion signal, FLAG sequence consensus and LysM-containing cA surface anchor (AcmA) ensuring cell-surface peptidoglycan anchoring. We demonstrate that all ILP variants are expressed in L. lactis cells, efficiently transported and secreted from the cell and displayed on the bacterial surface. The binding function of AcmA-immobilized ILP proteins is documented by interaction with a recombinant p19 protein, alpha subunit of human IL-23, which was assembled in the form of a fusion with Thioredoxin A. ILP317 variant exhibits the best binding to the human IL-23 cytokine, as demonstrated for particular L.lactis-ILP recombinant variants by Enzyme-Linked ImmunoSorbent Assay (ELISA). We conclude that novel recombinant ILP-secreting L. lactis strains were developed that might be useful for further in vivo studies of IL-23-mediated inflammation on animal model of experimentally-induced colitis.
Keywords: IL-23; albumin-binding domain; binding protein; cytokine; lactococcus; surface display.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting IL-6 by engineered Lactococcus lactis via surface-displayed affibody.Microb Cell Fact. 2022 Jul 16;21(1):143. doi: 10.1186/s12934-022-01873-7. Microb Cell Fact. 2022. PMID: 35842694 Free PMC article.
-
Engineered Lactococcus lactis Secreting IL-23 Receptor-Targeted REX Protein Blockers for Modulation of IL-23/Th17-Mediated Inflammation.Microorganisms. 2019 May 27;7(5):152. doi: 10.3390/microorganisms7050152. Microorganisms. 2019. PMID: 31137908 Free PMC article.
-
p19-targeted ABD-derived protein variants inhibit IL-23 binding and exert suppressive control over IL-23-stimulated expansion of primary human IL-17+ T-cells.Autoimmunity. 2017 Mar;50(2):102-113. doi: 10.1080/08916934.2016.1272598. Epub 2017 Jan 19. Autoimmunity. 2017. PMID: 28100093
-
Secretion and surface display of binders of IL-23/IL-17 cytokines and their receptors in Lactococcus lactis as a therapeutic approach against inflammation.Eur J Pharm Sci. 2023 Nov 1;190:106568. doi: 10.1016/j.ejps.2023.106568. Epub 2023 Aug 22. Eur J Pharm Sci. 2023. PMID: 37619953
-
Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion.J Mol Microbiol Biotechnol. 2008;14(1-3):48-58. doi: 10.1159/000106082. J Mol Microbiol Biotechnol. 2008. PMID: 17957110 Review.
Cited by
-
Dual Functionalized Lactococcus lactis Shows Tumor Antigen Targeting and Cytokine Binding in Vitro.Front Bioeng Biotechnol. 2022 Jan 26;10:822823. doi: 10.3389/fbioe.2022.822823. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35155394 Free PMC article.
-
Plasmid-Based Gene Expression Systems for Lactic Acid Bacteria: A Review.Microorganisms. 2022 May 31;10(6):1132. doi: 10.3390/microorganisms10061132. Microorganisms. 2022. PMID: 35744650 Free PMC article. Review.
-
Targeting IL-6 by engineered Lactococcus lactis via surface-displayed affibody.Microb Cell Fact. 2022 Jul 16;21(1):143. doi: 10.1186/s12934-022-01873-7. Microb Cell Fact. 2022. PMID: 35842694 Free PMC article.
-
Recent advances in bacteria-based platforms for inflammatory bowel diseases treatment.Exploration (Beijing). 2024 Mar 5;4(5):20230142. doi: 10.1002/EXP.20230142. eCollection 2024 Oct. Exploration (Beijing). 2024. PMID: 39439496 Free PMC article. Review.
-
Engineered bacteria as drug delivery vehicles: Principles and prospects.Eng Microbiol. 2022 Jun 21;2(3):100034. doi: 10.1016/j.engmic.2022.100034. eCollection 2022 Sep. Eng Microbiol. 2022. PMID: 39629029 Free PMC article. Review.
References
-
- Siakavellas S.I., Bamias G. Role of the IL-23/IL-17 axis in Crohn’s disease. Discov. Med. 2012;14:253–262. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

