Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma
- PMID: 29966520
- PMCID: PMC6027578
- DOI: 10.1186/s40425-018-0375-1
Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma
Abstract
Background: Metformin is one of the biguanides commonly used in patients with type II Diabetes Mellitus. Apart from its hypoglycemic properties, metformin also inhibits the cell cycle by restricting protein synthesis and cell proliferation via regulating the LKB1/AMPL pathway. Furthermore, it also enhances the PD-1 blockade through a reduction of tumor hypoxia. Metformin has shown a significant favorable impact on treatment-related outcomes in solid tumors, but these outcomes have not been replicated in the limited clinical studies done on malignant melanoma. Moreover, none of these studies have reported on the efficacy of the combined use of metformin and immune checkpoint inhibitors (ICIs).
Methods: This is a retrospective cohort study that includes patients diagnosed with metastatic malignant melanoma and treated with ipilimumab, nivolumab, and/or pembrolizumab (Cohort A); or ipilimumab, nivolumab, and/or pembrolizumab plus metformin (Cohort B) between January 1st 2011 through December 15th 2017. In this study, patients are stratified based on anti-PD-1 only and anti-CTLA4/anti-PD-1 combination therapies in each cohort. Objective response rate (ORR) is the primary endpoint. Disease control rate (DCR), overall survival (OS) and progression-free survival (PFS) are the secondary endpoints.
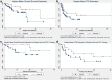
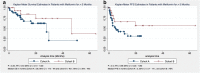
Results: Cohort A had 33 patients (60%), while cohort B had 22 (40%). Overall patient characteristics were similar between both cohorts. ORR was higher in cohort B (68.2% vs. 54.5%, P = 0.31). The DCR was higher in cohort B as well (77.3% vs. 60.6%, P = 0.19). Median OS (46.7 months vs. 28 months), and median PFS (19.8 months vs. 5 months) were longer in cohort B. However, on univariate and multivariate analyses, none of these differences were statistically significant. The mean number of new metastatic sites which appeared during therapy were significantly higher in cohort A (A:1.51 vs. B:0.59, P = 0.009).
Conclusion: We have observed favorable treatment-related outcomes (ORR, DCR, median PFS and median OS) in patients who have received metformin in combination with ICIs without reaching significance, probably, due to small sample size. Hence, large prospective clinical trials are required to study the synergistic effect of metformin in combination with ICIs before it can be recommended as routine additive therapy.
Keywords: Anti-PD-1/anti-CTLA-4; Ipilimumab; Malignant melanoma; Metformin; Nivolumab; Pembrolizumab.
Conflict of interest statement
Ethics approval and consent to participate
IRB approval was obtained before initiation of the study activities. Due to retrospective nature of the study, request for waiver from patient’s consent was approved by IRB. IRB approval letter can be requested from the corresponding author.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Rang HP. Pharmacology. 5. New York: Churchill Livingstone; 2003. p. 388.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical