Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis
- PMID: 29966587
- PMCID: PMC6029847
- DOI: 10.7554/eLife.36354
Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis
Abstract
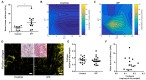
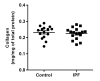
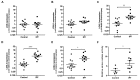
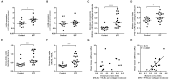
Matrix stiffening with downstream activation of mechanosensitive pathways is strongly implicated in progressive fibrosis; however, pathologic changes in extracellular matrix (ECM) that initiate mechano-homeostasis dysregulation are not defined in human disease. By integrated multiscale biomechanical and biological analyses of idiopathic pulmonary fibrosis lung tissue, we identify that increased tissue stiffness is a function of dysregulated post-translational collagen cross-linking rather than any collagen concentration increase whilst at the nanometre-scale collagen fibrils are structurally and functionally abnormal with increased stiffness, reduced swelling ratio, and reduced diameter. In ex vivo and animal models of lung fibrosis, dual inhibition of lysyl oxidase-like (LOXL) 2 and LOXL3 was sufficient to normalise collagen fibrillogenesis, reduce tissue stiffness, and improve lung function in vivo. Thus, in human fibrosis, altered collagen architecture is a key determinant of abnormal ECM structure-function, and inhibition of pyridinoline cross-linking can maintain mechano-homeostasis to limit the self-sustaining effects of ECM on progressive fibrosis.
Keywords: biomechanics; collagen; extracellular matrix; fibrosis; human; human biology; lung; lysyl oxidase; medicine; mouse.
© 2018, Jones et al.
Conflict of interest statement
MJ, OA, KA, DS, AB, PJ, AA, FC, RD, CL, BJ, KB, SF, BM, SJ, CB, SC, CO, PS, JG, MK, SM, AF, AB, LR, KO, PT No competing interests declared, JR As a current employee of Synairgen Research Ltd, J.J.W.R has share options in the company. KL As a current employee of Synairgen Research Ltd, K.L has share options in the company. VT As a current employee of Synairgen Research Ltd, V.J.T has share options in the company. LC As a former or current employee of Pharmaxis Pharmaceuticals Ltd, L.C. has an equity stake in the company. WJ As a current employee of Pharmaxis Pharmaceuticals Ltd, W.J. has an equity stake in the company. PM As a current employee of Synairgen Research Ltd, P.D.M has share options and is a share-holder in the company. DD D.E.D is co-founder of, share-holder in and consultant to Synairgen Research Ltd.
Figures

Similar articles
-
Lysyl Oxidase-Like 1 Protein Deficiency Protects Mice from Adenoviral Transforming Growth Factor-β1-induced Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2018 Apr;58(4):461-470. doi: 10.1165/rcmb.2017-0252OC. Am J Respir Cell Mol Biol. 2018. PMID: 29115860
-
Extracellular Matrix Cross-Linking Enhances Fibroblast Growth and Protects against Matrix Proteolysis in Lung Fibrosis.Am J Respir Cell Mol Biol. 2018 May;58(5):594-603. doi: 10.1165/rcmb.2016-0379OC. Am J Respir Cell Mol Biol. 2018. PMID: 29053339
-
TGFβ-1 Induced Cross-Linking of the Extracellular Matrix of Primary Human Dermal Fibroblasts.Int J Mol Sci. 2021 Jan 20;22(3):984. doi: 10.3390/ijms22030984. Int J Mol Sci. 2021. PMID: 33498156 Free PMC article.
-
Collagen cross-linking mediated by lysyl hydroxylase 2: an enzymatic battlefield to combat fibrosis.Essays Biochem. 2019 Sep 13;63(3):377-387. doi: 10.1042/EBC20180051. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31324706 Review.
-
LOX/LOXL in pulmonary fibrosis: potential therapeutic targets.J Drug Target. 2019 Aug;27(7):790-796. doi: 10.1080/1061186X.2018.1550649. Epub 2018 Nov 29. J Drug Target. 2019. PMID: 30457362 Review.
Cited by
-
Role of Collagen in Airway Mechanics.Bioengineering (Basel). 2021 Jan 16;8(1):13. doi: 10.3390/bioengineering8010013. Bioengineering (Basel). 2021. PMID: 33467161 Free PMC article. Review.
-
Perivascular cells function as key mediators of mechanical and structural changes in vascular capillaries.Sci Adv. 2025 Jan 10;11(2):eadp3789. doi: 10.1126/sciadv.adp3789. Epub 2025 Jan 10. Sci Adv. 2025. PMID: 39792671 Free PMC article.
-
Mechanical and metabolic interplay in the brain metastatic microenvironment.Front Oncol. 2022 Aug 18;12:932285. doi: 10.3389/fonc.2022.932285. eCollection 2022. Front Oncol. 2022. PMID: 36059679 Free PMC article.
-
Periadventitial β-aminopropionitrile-loaded nanofibers reduce fibrosis and improve arteriovenous fistula remodeling in rats.Front Cardiovasc Med. 2023 Feb 28;10:1124106. doi: 10.3389/fcvm.2023.1124106. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36926045 Free PMC article.
-
How collagen becomes 'stiff'.Elife. 2022 Feb 21;11:e77041. doi: 10.7554/eLife.77041. Elife. 2022. PMID: 35188461 Free PMC article.
References
-
- Andriotis OG, Manuyakorn W, Zekonyte J, Katsamenis OL, Fabri S, Howarth PH, Davies DE, Thurner PJ. Nanomechanical assessment of human and murine collagen fibrils via atomic force microscopy cantilever-based nanoindentation. Journal of the Mechanical Behavior of Biomedical Materials. 2014;39:9–26. doi: 10.1016/j.jmbbm.2014.06.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

