Post-translational modifications of transporters
- PMID: 29966598
- PMCID: PMC6263853
- DOI: 10.1016/j.pharmthera.2018.06.013
Post-translational modifications of transporters
Abstract
Drug transporter proteins are critical to the distribution of a wide range of endogenous compounds and xenobiotics such as hormones, bile acids, peptides, lipids, sugars, and drugs. There are two classes of drug transporters- the solute carrier (SLC) transporters and ATP-binding cassette (ABC) transporters -which predominantly differ in the energy source utilized to transport substrates across a membrane barrier. Despite their hydrophobic nature and residence in the membrane bilayer, drug transporters have dynamic structures and adopt many conformations during the translocation process. Whereas there is significant literature evidence for the substrate specificity and structure-function relationship for clinically relevant drug transporters proteins, there is less of an understanding in the regulatory mechanisms that contribute to the functional expression of these proteins. Post-translational modifications have been shown to modulate drug transporter functional expression via a wide range of molecular mechanisms. These modifications commonly occur through the addition of a functional group (e.g. phosphorylation), a small protein (e.g. ubiquitination), sugar chains (e.g. glycosylation), or lipids (e.g. palmitoylation) on solvent accessible amino acid residues. These covalent additions often occur as a result of a signaling cascade and may be reversible depending on the type of modification and the intended fate of the signaling event. Here, we review the significant role in which post-translational modifications contribute to the dynamic regulation and functional consequences of SLC and ABC drug transporters and highlight recent progress in understanding their roles in transporter structure, function, and regulation.
Keywords: Glycosylation; Membrane transport; Palmitoylation; Phosphorylation; Post-translational modification; SUMOylation; Ubiquitination.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
LCC and PWS declare no conflicts of interest. KMH is an employee of Eli Lilly & Company.
Figures
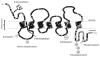
References
-
- Cesar-Razquin A Snijder B Frappier-Brinton T Isserlin R Gyimesi G Bai X et al. A Call for Systematic Research on Solute Carriers Cell 2015. 162 3 478–87 - PubMed
-
- Harris NJ Booth PJ Folding and stability of membrane transport proteins in vitro Biochim Biophys Acta 2012. 1818 4 1055–66 - PubMed
-
- Harris NJ Findlay HE Simms J Liu X Booth PJ Relative domain folding and stability of a membrane transport protein J Mol Biol 2014. 426 8 1812–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

