Hedgehog signaling from the Posterior Signaling Center maintains U-shaped expression and a prohemocyte population in Drosophila
- PMID: 29966604
- PMCID: PMC6064674
- DOI: 10.1016/j.ydbio.2018.06.020
Hedgehog signaling from the Posterior Signaling Center maintains U-shaped expression and a prohemocyte population in Drosophila
Abstract
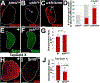
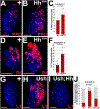
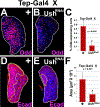
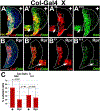
Hematopoietic progenitor choice between multipotency and differentiation is tightly regulated by intrinsic factors and extrinsic signals from the surrounding microenvironment. The Drosophila melanogaster hematopoietic lymph gland has emerged as a powerful tool to investigate mechanisms that regulate hematopoietic progenitor choice in vivo. The lymph gland contains progenitor cells, which share key characteristics with mammalian hematopoietic progenitors such as quiescence, multipotency and niche-dependence. The lymph gland is zonally arranged, with progenitors located in medullary zone, differentiating cells in the cortical zone, and the stem cell niche or Posterior Signaling Center (PSC) residing at the base of the medullary zone (MZ). This arrangement facilitates investigations into how signaling from the microenvironment controls progenitor choice. The Drosophila Friend of GATA transcriptional regulator, U-shaped, is a conserved hematopoietic regulator. To identify additional novel intrinsic and extrinsic regulators that interface with U-shaped to control hematopoiesis, we conducted an in vivo screen for factors that genetically interact with u-shaped. Smoothened, a downstream effector of Hedgehog signaling, was one of the factors identified in the screen. Here we report our studies that characterized the relationship between Smoothened and U-shaped. We showed that the PSC and Hedgehog signaling are required for U-shaped expression and that U-shaped is an important intrinsic progenitor regulator. These observations identify a potential link between the progenitor regulatory machinery and extrinsic signals from the PSC. Furthermore, we showed that both Hedgehog signaling and the PSC are required to maintain a subpopulation of progenitors. This led to a delineation of PSC-dependent versus PSC-independent progenitors and provided further evidence that the MZ progenitor population is heterogeneous. Overall, we have identified a connection between a conserved hematopoietic master regulator and a putative stem cell niche, which adds to our understanding of how signals from the microenvironment regulate progenitor multipotency.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

