Intracellular emetic signaling evoked by the L-type Ca2+ channel agonist FPL64176 in the least shrew (Cryptotis parva)
- PMID: 29966616
- PMCID: PMC6104632
- DOI: 10.1016/j.ejphar.2018.06.035
Intracellular emetic signaling evoked by the L-type Ca2+ channel agonist FPL64176 in the least shrew (Cryptotis parva)
Abstract
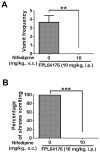
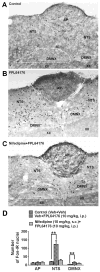
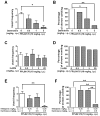
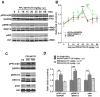
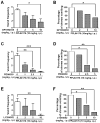
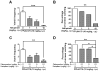
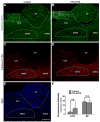
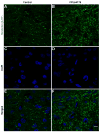
Ca2+ plays a major role in maintaining cellular homeostasis and regulates processes including apoptotic cell death and side-effects of cancer chemotherapy including vomiting. Currently we explored the emetic mechanisms of FPL64176, an L-type Ca2+ channel (LTCC) agonist with maximal emetogenic effect at its 10 mg/kg dose. FPL64176 evoked c-Fos immunoreactivity in shrew brainstem sections containing the vomit-associated nuclei, nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus. FPL64176 also increased phosphorylation of proteins ERK1/2, PKCα/βII and Akt in the brainstem. Moreover, their corresponding inhibitors (PD98059, GF 109203X and LY294002, respectively) reduced FPL64176-evoked vomiting. A 30 min subcutaneous (s.c.) pretreatment with the LTCC antagonist nifedipine (10 mg/kg) abolished FPL64176-elicited vomiting, c-Fos expression, and emetic effector phosphorylation. Ryanodine receptors (RyRs) and inositol trisphosphate receptors (IP3Rs) mediate intracellular Ca2+ release from the sarcoplasmic/endoplasmic reticulum. The RyR antagonist dantrolene (i.p.), or a combination of low doses of nifedipine and dantrolene, but not the IP3R antagonist 2-APB, significantly attenuated FPL64176-induced vomiting. The serotonin type 3 receptor (5-HT3R) antagonist palonosetron (s.c.), the neurokinin 1 receptor (NK1R) antagonist netupitant (i.p.) or a combination of non-effective doses of netupitant and palonosetron showed antiemetic potential against FPL64176-evoked vomiting. Serotonin (5-HT) and substance P immunostaining revealed FPL64176-induced emesis was accompanied by an increase in 5-HT but not SP-immunoreactivity in the dorsomedial subdivision of the NTS. These findings demonstrate that Ca2+ mobilization through LTCCs and RyRs, and subsequent emetic effector phosphorylation and 5-HT release play important roles in FPL64176-induced emesis which can be prevented by 5-HT3R and NK1R antagonists.
Keywords: ERK; FPL64176; L-type Ca(2+) channel; Ryanodine receptor; Serotonin.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
References
-
- Beltran-Parrazal L, Fernandez-Ruiz J, Toledo R, Manzo J, Morgado-Valle C. Inhibition of endoplasmic reticulum Ca2+ ATPase in preBötzinger complex of neonatal rat does not affect respiratory rhythm generation. Neuroscience. 2012;224:116–124. - PubMed
-
- Bjarkam CR, Sørensen JC, Geneser FA. Distribution and morphology of serotonin-immunoreactive axons in the hippocampal region of the New Zealand white rabbit. I Area dentata and hippocampus. Hippocampus. 2003;13:21–37. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

