Nicotine induces oral dysplastic keratinocyte migration via fatty acid synthase-dependent epidermal growth factor receptor activation
- PMID: 29966661
- PMCID: PMC6108942
- DOI: 10.1016/j.yexcr.2018.06.036
Nicotine induces oral dysplastic keratinocyte migration via fatty acid synthase-dependent epidermal growth factor receptor activation
Abstract
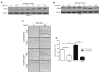
Despite advances in diagnostic and therapeutic management, oral squamous cell carcinoma (OSCC) patient survival rates have remained relatively unchanged. Thus, identifying early triggers of malignant progression is critical to prevent OSCC development. Traditionally, OSCC initiation is elicited by the frequent and direct exposure to multiple tobacco-derived carcinogens, and not by the nicotine contained in tobacco products. However, other nicotine-containing products, especially the increasingly popular electronic cigarettes (e-cigs), have unknown effects on the progression of undiagnosed tobacco-induced oral premalignant lesions, specifically in regard to the effects of nicotine. Overexpression of fatty acid synthase (FASN), a key hepatic de novo lipogenic enzyme, is linked to poor OSCC patient survival. Nicotine upregulates hepatic FASN, but whether this response occurs in oral dysplastic keratinocytes is unknown. We hypothesized that in oral dysplastic keratinocytes, nicotine triggers a migratory phenotype through FASN-dependent epidermal growth factor receptor (EGFR) activation, a common pro-oncogenic event supporting oral carcinogenesis. We report that in oral dysplastic cells, nicotine markedly upregulates FASN leading to FASN-dependent EGFR activation and increased cell migration. These results raise potential concerns about e-cig safety, especially when used by former tobacco smokers with occult oral premalignant lesions where nicotine could trigger oncogenic signals commonly associated with malignant progression.
Keywords: Cell migration; EGFR; FASN; Nicotine; Oral cancer; Oral premalignant lesions.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures








References
-
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabe E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castaneda-Orjuela C, Catala-Lopez F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, GHTT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Soreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabares-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncology. 2017;3:524–548. - PMC - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Wisniewski DJ, Ma T, Schneider A. Advances in the Chemopreventive Targeting of Oral Carcinogenesis. Current Oral Health Reports. 2015;2:63–72.
-
- Amagasa T, Yamashiro M, Uzawa N. Oral premalignant lesions: from a clinical perspective. International Journal of Clinical Oncology. 2011;16:5–14. - PubMed
-
- Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States. 2014, NCHS data brief. 2015:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

