Translation of Clinical Research into Practice: An Impact Assessment of the Results from the Blood and Marrow Transplant Clinical Trials Network Protocol 0201 on Unrelated Graft Source Utilization
- PMID: 29966761
- PMCID: PMC6242749
- DOI: 10.1016/j.bbmt.2018.06.028
Translation of Clinical Research into Practice: An Impact Assessment of the Results from the Blood and Marrow Transplant Clinical Trials Network Protocol 0201 on Unrelated Graft Source Utilization
Abstract
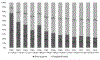
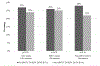
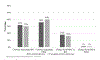
Barriers and facilitators to adoption of results of clinical trials are substantial and poorly understood. We sought to examine whether the results of the randomized, multicenter Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0201 study comparing peripheral blood (PB) with bone marrow (BM) stem cells for unrelated donor (URD) hematopoietic cell transplantation (HCT) changed practice from PB to BM graft utilization and explored factors that impact graft selection and translation of research results into practice. The difference between use of URD BM and PB in the 2 years before and after publication of results in 2012 was examined using observational data collected by the Center for Blood and Marrow Transplant Research. A web-based survey of transplant physicians was conducted to understand the change in physician-reported personal and center preferred URD graft. No significant change in use of BM versus PB grafts occurred after 2012. Both BMT CTN participating and nonparticipating centers continued to use PB. Ninety-two percent of respondents were aware of the study results; 18% reported a change in personal and 16% reported a change in their center's practice of requesting BM instead of PB for URD HCT. Patient characteristics and the perception that engaging local champions to increase the evidence uptake were factors associated with personal or center change in practice. Despite awareness of the trial results, fewer than one-fifth of HCT physicians reported practice change in response to the BMT CTN 0201 results. Observational data confirmed no discernible change in practice.
Keywords: Evidence-based practice; Hematopoietic cell transplantation; Practice patterns; Randomized clinical trials.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest: The authors have no relevant financial conflicts of interest to disclose.
Figures

References
-
- Lamas GA, Pfeffer MA, Hamm P, Wertheimer J, Rouleau JL, Braunwald E. Do the results of randomized clinical trials of cardiovascular drugs influence medical practice? The SAVE Investigators. The New England journal of medicine. 1992;327:241–247. - PubMed
-
- McKenna CJ, Codd MB, McCann HA, Sugrue DD. International trials and national practice: A questionnaire survey of current physician practice in the treatment of acute myocardial infarction. I.J.M.S 1996;165:157–158. - PubMed
-
- Omoigui NA, Silver MJ, Rybicki LA, et al. Influence of a Randomized Clinical Trial on Practice by Participating Investigators: Lessons From the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT) fn1. Journal of the American College of Cardiology. 1998;31:265–272. - PubMed
-
- Ketley D, Woods KL. Impact of clinical trials on clinical practice: example of thrombolysis for acute myocardial infarction. The Lancet. 1993;342:891–894. - PubMed
-
- Stafford RS, Furberg CD, Finkelstein SN, Cockburn IM, Alehegn T, Ma J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996-2002. JAMA : the journal of the American Medical Association. 2004;291:54–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

