Structural and functional analysis of cystatin E reveals enzymologically relevant dimer and amyloid fibril states
- PMID: 29967063
- PMCID: PMC6109925
- DOI: 10.1074/jbc.RA118.002154
Structural and functional analysis of cystatin E reveals enzymologically relevant dimer and amyloid fibril states
Abstract
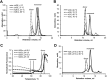
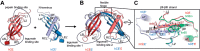
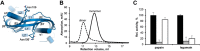
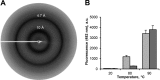
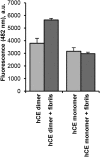
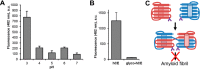
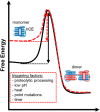
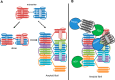
Protein activity is often regulated by altering the oligomerization state. One mechanism of multimerization involves domain swapping, wherein proteins exchange parts of their structures and thereby form long-lived dimers or multimers. Domain swapping has been specifically observed in amyloidogenic proteins, for example the cystatin superfamily of cysteine protease inhibitors. Cystatins are twin-headed inhibitors, simultaneously targeting the lysosomal cathepsins and legumain, with important roles in cancer progression and Alzheimer's disease. Although cystatin E is the most potent legumain inhibitor identified so far, nothing is known about its propensity to oligomerize. In this study, we show that conformational destabilization of cystatin E leads to the formation of a domain-swapped dimer with increased conformational stability. This dimer was active as a legumain inhibitor by forming a trimeric complex. By contrast, the binding sites toward papain-like proteases were buried within the cystatin E dimer. We also showed that the dimers could further convert to amyloid fibrils. Unexpectedly, cystatin E amyloid fibrils contained functional protein, which inhibited both legumain and papain-like enzymes. Fibril formation was further regulated by glycosylation. We speculate that cystatin amyloid fibrils might serve as a binding platform to stabilize the pH-sensitive legumain and cathepsins in the extracellular environment, contributing to their physiological and pathological functions.
Keywords: amyloid; conformational change; cysteine protease; enzyme inhibitor; protein stability; protein structure.
© 2018 Dall et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Abrahamson M., Barrett A. J., Salvesen G., and Grubb A. (1986) Isolation of six cysteine proteinase inhibitors from human urine: their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J. Biol. Chem. 261, 11282–11289 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

