Plasmodium vivax Merozoite Surface Protein 1 Paralog as a Mediator of Parasite Adherence to Reticulocytes
- PMID: 29967091
- PMCID: PMC6105898
- DOI: 10.1128/IAI.00239-18
Plasmodium vivax Merozoite Surface Protein 1 Paralog as a Mediator of Parasite Adherence to Reticulocytes
Abstract
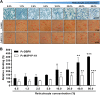
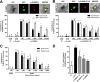
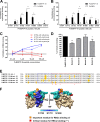
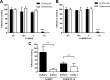
Plasmodium vivax parasites preferentially invade reticulocytes in human beings. P. vivax merozoite surface protein 1 (PvMSP1) and PvMSP1 paralog (PvMSP1P) may have important functions in reticulocyte adherence during invasion. These proteins share similar structures, including the presence of two epidermal growth factor (EGF)-like and glycosylphosphatidylinositol (GPI)-anchored domains at the C terminus. However, there have been no reports concerning the functional activity of PvMSP1P in reticulocyte adherence during P. vivax invasion. In this study, the ability of PvMSP1P-19 to bind to reticulocytes and normocytes was analyzed. The reticulocyte binding activity of PvMSP1P-19 was 4.0-fold higher than its normocyte binding activity. The binding of PvMSP1P-19 to reticulocytes and normocytes was inhibited in a dose-dependent manner by antibodies from immunized rabbits and by antibodies from vivax parasite-infected patients. Consistently, antibodies against PvMSP1P inhibited parasite invasion during short-term in vitro cultivation. Similar to the case for PvDBPII binding activity, PvMSP1P-19 binding activity was reduced in chymotrypsin-treated reticulocytes. However, no significant difference between the binding of PvMSP1P-19 to Duffy-positive and Duffy-negative erythrocytes was found. The minimal binding motif of PvMSP1P-19 was characterized using synthetic peptides. The results showed that the residues at amino acid positions 1791 to 1808 may have an important function in mediating merozoite adherence to reticulocytes. The positively charged residues within the EGF-like domain were shown to constitute a key binding motif. This work presents strong evidence supporting the role of PvMSP1P in host target cell selection and invasion of Duffy-independent pathway in P. vivax Moreover, PvMSP1P-19-specific antibodies may confer protection against P. vivax reinvasion.
Keywords: Duffy independent; Plasmodium vivax; invasion inhibition; merozoite surface; merozoite surface protein 1; merozoite surface protein 1 paralog; reticulocyte.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- World Health Organization. 2017. World malaria report 2016. World Health Organization, Geneva, Switzerland.
-
- Malleret B, Xu F, Mohandas N, Suwanarusk R, Chu C, Leite JA, Low K, Turner C, Sriprawat K, Zhang R, Bertrand O, Colin Y, Costa FT, Ong CN, Ng ML, Lim CT, Nosten F, Renia L, Russell B. 2013. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS One 8:e76062. doi: 10.1371/journal.pone.0076062. - DOI - PMC - PubMed
-
- Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, Bertrand O, Picot J, King CL, Grimberg BT, Mercereau-Puijalon O, Zimmerman PA. 2010. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A 107:5967–5971. doi: 10.1073/pnas.0912496107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

