Decoding on the ribosome depends on the structure of the mRNA phosphodiester backbone
- PMID: 29967153
- PMCID: PMC6055197
- DOI: 10.1073/pnas.1721431115
Decoding on the ribosome depends on the structure of the mRNA phosphodiester backbone
Abstract
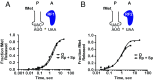
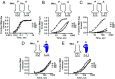
During translation, the ribosome plays an active role in ensuring that mRNA is decoded accurately and rapidly. Recently, biochemical studies have also implicated certain accessory factors in maintaining decoding accuracy. However, it is currently unclear whether the mRNA itself plays an active role in the process beyond its ability to base pair with the tRNA. Structural studies revealed that the mRNA kinks at the interface of the P and A sites. A magnesium ion appears to stabilize this structure through electrostatic interactions with the phosphodiester backbone of the mRNA. Here we examined the role of the kink structure on decoding using a well-defined in vitro translation system. Disruption of the kink structure through site-specific phosphorothioate modification resulted in an acute hyperaccurate phenotype. We measured rates of peptidyl transfer for near-cognate tRNAs that were severely diminished and in some instances were almost 100-fold slower than unmodified mRNAs. In contrast to peptidyl transfer, the modifications had little effect on GTP hydrolysis by elongation factor thermal unstable (EF-Tu), suggesting that only the proofreading phase of tRNA selection depends critically on the kink structure. Although the modifications appear to have no effect on typical cognate interactions, peptidyl transfer for a tRNA that uses atypical base pairing is compromised. These observations suggest that the kink structure is important for decoding in the absence of Watson-Crick or G-U wobble base pairing at the third position. Our findings provide evidence for a previously unappreciated role for the mRNA backbone in ensuring uniform decoding of the genetic code.
Keywords: decoding; mRNA structure; phosphorothioate substitution; ribosome; tRNA selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




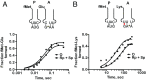
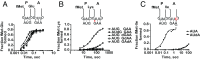
References
-
- Rosenberger RF, Foskett G. An estimate of the frequency of in vivo transcriptional errors at a nonsense codon in Escherichia coli. Mol Gen Genet. 1981;183:561–563. - PubMed
-
- Edelmann P, Gallant J. Mistranslation in E. coli. Cell. 1977;10:131–137. - PubMed
-
- Thompson RC. EFTu provides an internal kinetic standard for translational accuracy. Trends Biochem Sci. 1988;13:91–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

