Autophagy defects and related genetic variations in renal cell carcinoma with eosinophilic cytoplasmic inclusions
- PMID: 29967346
- PMCID: PMC6028630
- DOI: 10.1038/s41598-018-28369-y
Autophagy defects and related genetic variations in renal cell carcinoma with eosinophilic cytoplasmic inclusions
Abstract
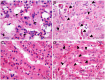
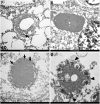
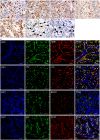
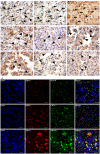
The relationship between autophagy and tumour is well studied, but tumour cell morphological changes associated with autophagy defects are rarely reported, especially in renal cell carcinoma (RCC). We collected 10 renal tumour samples with characteristic eosinophilic cytoplasmic inclusions (ECIs) and found that the ECIs were majorly composed of sequestosome 1/P62, neighbor of BRCA1 gene 1 (NBR1), PEX14, and CATALASE1 (CAT1). Further, transmission electron microscopy analysis revealed that ECIs were aggregates of proteinaceous material and peroxisomes. These results confirmed that ECIs in RCCs were the products of autophagy defects. The presence of ECIs was correlated with high Fuhrman grade components of RCCs. Whole-exome sequencing (WES) and Sanger sequencing confirmed that tumours with ECIs showed somatic mutations or high frequency of genetic variations in autophagy-related (ATG) genes, such as ATG7, ATG5, and ATG10. These results indicate that nucleotide changes in ATG genes are associated with autophagy defect, ECI formation, and even tumour grade in RCCs.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Unger P, et al. Eosinophilic globules resembling Mallory bodies in a renal cell carcinoma. N Y State J Med. 1992;92:18–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

