Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway
- PMID: 29967419
- PMCID: PMC6028641
- DOI: 10.1038/s41467-018-04985-0
Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway
Abstract
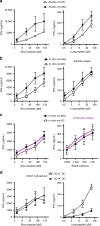
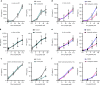
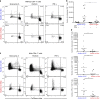
Presentation of exogenous antigens on MHC-I molecules, termed cross-presentation, is essential for cytotoxic CD8+ T cell responses. In mice, dendritic cells (DCs) that arise from monocytes (mo-DCs) during inflammation have a key function in these responses by cross-presenting antigens locally in peripheral tissues. Whether human naturally-occurring mo-DCs can cross-present is unknown. Here, we use human mo-DCs and macrophages directly purified from ascites to address this question. Single-cell RNA-seq data show that ascites CD1c+ DCs contain exclusively monocyte-derived cells. Both ascites mo-DCs and monocyte-derived macrophages cross-present efficiently, but are inefficient for transferring exogenous proteins into their cytosol. Inhibition of cysteine proteases, but not of proteasome, abolishes cross-presentation in these cells. We conclude that human monocyte-derived cells cross-present exclusively using a vacuolar pathway. Finally, only ascites mo-DCs provide co-stimulatory signals to induce effector cytotoxic CD8+ T cells. Our findings thus provide important insights on how to harness cross-presentation for therapeutic purposes.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
IL-10 promotes homeostatic proliferation of human CD8(+) memory T cells and, when produced by CD1c(+) DCs, shapes naive CD8(+) T-cell priming.Eur J Immunol. 2016 Jul;46(7):1622-32. doi: 10.1002/eji.201546136. Epub 2016 May 17. Eur J Immunol. 2016. PMID: 27129615
-
Different antigen-processing activities in dendritic cells, macrophages, and monocytes lead to uneven production of HIV epitopes and affect CTL recognition.J Immunol. 2014 Nov 1;193(9):4322-4334. doi: 10.4049/jimmunol.1400491. Epub 2014 Sep 17. J Immunol. 2014. PMID: 25230751 Free PMC article.
-
Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells.J Exp Med. 2013 May 6;210(5):1035-47. doi: 10.1084/jem.20121103. Epub 2013 Apr 8. J Exp Med. 2013. PMID: 23569327 Free PMC article.
-
Antigen Cross-Presentation by Macrophages.Front Immunol. 2020 Jul 8;11:1276. doi: 10.3389/fimmu.2020.01276. eCollection 2020. Front Immunol. 2020. PMID: 32733446 Free PMC article. Review.
-
Antigen presentation by mouse monocyte-derived cells: Re-evaluating the concept of monocyte-derived dendritic cells.Mol Immunol. 2021 Jul;135:165-169. doi: 10.1016/j.molimm.2021.04.012. Epub 2021 Apr 23. Mol Immunol. 2021. PMID: 33901761 Review.
Cited by
-
Deciphering the TCR Repertoire to Solve the COVID-19 Mystery.Trends Pharmacol Sci. 2020 Aug;41(8):518-530. doi: 10.1016/j.tips.2020.06.001. Epub 2020 Jun 20. Trends Pharmacol Sci. 2020. PMID: 32576386 Free PMC article. Review.
-
Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines?Front Immunol. 2018 Dec 13;9:2874. doi: 10.3389/fimmu.2018.02874. eCollection 2018. Front Immunol. 2018. PMID: 30619259 Free PMC article. Review.
-
Unexpected Role of CD8 T Cells in Accelerated Clearance of Salmonella enterica Serovar Typhimurium from H-2 Congenic mice.Infect Immun. 2019 Oct 18;87(11):e00588-19. doi: 10.1128/IAI.00588-19. Print 2019 Nov. Infect Immun. 2019. PMID: 31427450 Free PMC article.
-
Tissue-Resident Immune Cells in Humans.Annu Rev Immunol. 2022 Apr 26;40:195-220. doi: 10.1146/annurev-immunol-093019-112809. Epub 2022 Jan 19. Annu Rev Immunol. 2022. PMID: 35044795 Free PMC article. Review.
-
Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states.Nat Commun. 2022 Jan 10;13(1):141. doi: 10.1038/s41467-021-27322-4. Nat Commun. 2022. PMID: 35013146 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

