Different concentrations of lipopolysaccharide regulate barrier function through the PI3K/Akt signalling pathway in human pulmonary microvascular endothelial cells
- PMID: 29967433
- PMCID: PMC6028427
- DOI: 10.1038/s41598-018-28089-3
Different concentrations of lipopolysaccharide regulate barrier function through the PI3K/Akt signalling pathway in human pulmonary microvascular endothelial cells
Abstract
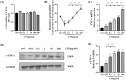
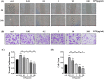
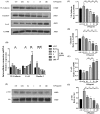
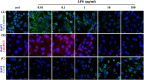
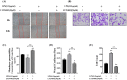
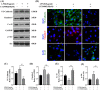
Lipopolysaccharide (LPS) can lead to vascular endothelial barrier dysfunction, which often results in acute lung injury and acute respiratory distress syndrome. However, the effects of different concentrations of LPS on human pulmonary microvascular endothelial barrier function and the involvement of the phosphatidylinositol-3-kinase-serine/threonine kinase (PI3K/Akt) pathway in this process remain unclear. Human pulmonary microvascular endothelial cells (HPMECs) were stimulated with different doses of LPS, and barrier function was examined by determining cell monolayer permeability, cell migration, and the expression of intercellular junction proteins (VE-Cadherin, Claudin-5, and Connexin-43). LY294002 was used to inhibit PI3K to verify the role of the PI3K/Akt pathway in the regulation of barrier function in HPMECs stimulated by LPS. Low doses of LPS increased HPMEC migration, up-regulated VE-Cadherin and Claudin-5 expression, down-regulated Connexin-43 expression, and promoted Akt phosphorylation, which could collectively decrease monolayer permeability. In contrast, high doses of LPS suppressed HPMEC migration, down-regulated the expression of VE-Cadherin and Claudin-5, up-regulated Connexin-43 expression, and reduced Akt phosphorylation, which could collectively increase monolayer permeability. LPS has a biphasic effect on HPMEC barrier function through the PI3K/Akt pathway, and this effect is concentration-dependent.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Force ADT, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81101445/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81101445/National Natural Science Foundation of China (National Science Foundation of China)/International
- LY16H150002/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)/International
- LY16H150002/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

