The lifelong impact of fetal growth restriction on cardiac development
- PMID: 29967522
- PMCID: PMC6265071
- DOI: 10.1038/s41390-018-0069-x
The lifelong impact of fetal growth restriction on cardiac development
Abstract
Background: Maternal nutrient restriction (MNR) is a widespread cause of fetal growth restriction (FGR), an independent predictor of heart disease and cardiovascular mortality. Our objective was to examine the developmental and long-term impact of MNR-induced FGR on cardiac structure in a model that closely mimics human development.
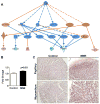
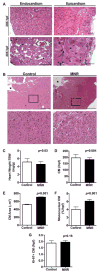
Methods: A reduction in total caloric intake spanning pregestation through to lactation in guinea pig sows was used to induce FGR. Proliferation, differentiation, and apoptosis of cardiomyocytes were assessed in late-gestation fetal, neonatal, and adult guinea pig hearts. Proteomic analysis and pathway enrichment were performed on fetal hearts.
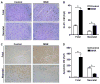
Results: Cardiomyocyte proliferation and the number of mononucleated cells were enhanced in the MNR-FGR fetal and neonatal heart, suggesting a delay in cardiomyocyte differentiation. In fetal hearts of MNR-FGR animals, apoptosis was markedly elevated and the total number of cardiomyocytes reduced, the latter remaining so throughout neonatal and into adult life. A reduction in total cardiomyocyte number in adult MNR-FGR hearts was accompanied by exaggerated hypertrophy and a disorganized architecture. Pathway analysis identified genes related to cell proliferation, differentiation, and survival.
Conclusions: FGR influences cardiomyocyte development during critical windows of development, leading to a permanent deficiency in cardiomyocyte number and compensatory hypertrophy in a rodent model that recapitulates human development.
Conflict of interest statement
Figures



Similar articles
-
Maternal nutrient restriction in guinea pigs as an animal model for studying growth-restricted offspring with postnatal catch-up growth.Am J Physiol Regul Integr Comp Physiol. 2018 May 1;314(5):R647-R654. doi: 10.1152/ajpregu.00317.2017. Epub 2018 Jan 10. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29351419
-
Maternal Nutrient Restriction in Guinea Pigs as an Animal Model for Inducing Fetal Growth Restriction.Reprod Sci. 2016 Feb;23(2):219-27. doi: 10.1177/1933719115602773. Epub 2015 Sep 3. Reprod Sci. 2016. PMID: 26342049
-
Maternal nutrient restriction in guinea pigs leads to fetal growth restriction with increased brain apoptosis.Pediatr Res. 2019 Jan;85(1):105-112. doi: 10.1038/s41390-018-0230-6. Epub 2018 Nov 12. Pediatr Res. 2019. PMID: 30420709
-
Fetal Growth Restriction: Does an Integrated Maternal Hemodynamic-Placental Model Fit Better?Reprod Sci. 2021 Sep;28(9):2422-2435. doi: 10.1007/s43032-020-00393-2. Epub 2020 Nov 19. Reprod Sci. 2021. PMID: 33211274 Free PMC article. Review.
-
Intrauterine Intervention for the Treatment of Fetal Growth Restriction.Curr Pediatr Rev. 2016;12(3):168-178. doi: 10.2174/1573396312666160808151856. Curr Pediatr Rev. 2016. PMID: 27515036 Review.
Cited by
-
Proportionality at birth and left ventricular hypertrophy in healthy adolescents.Early Hum Dev. 2019 May;132:24-29. doi: 10.1016/j.earlhumdev.2019.03.018. Epub 2019 Apr 3. Early Hum Dev. 2019. PMID: 30953878 Free PMC article.
-
Relationship between Abnormal Placenta and Obstetric Outcomes: A Meta-Analysis.Biomedicines. 2023 May 25;11(6):1522. doi: 10.3390/biomedicines11061522. Biomedicines. 2023. PMID: 37371617 Free PMC article. Review.
-
Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar-Rat Offspring.Nutrients. 2021 Jan 30;13(2):466. doi: 10.3390/nu13020466. Nutrients. 2021. PMID: 33573223 Free PMC article.
-
The association of low body mass index with neonatal morbidities in preterm infants.Sci Rep. 2021 Sep 22;11(1):18841. doi: 10.1038/s41598-021-98338-5. Sci Rep. 2021. PMID: 34552171 Free PMC article.
-
Fetal growth restriction and stillbirth: Biomarkers for identifying at risk fetuses.Front Physiol. 2022 Aug 19;13:959750. doi: 10.3389/fphys.2022.959750. eCollection 2022. Front Physiol. 2022. PMID: 36060697 Free PMC article. Review.
References
-
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. - PubMed
-
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. - PubMed
-
- Jiang B, Godfrey KM, Martyn CN, Gale CR. Birth weight and cardiac structure in children. Pediatrics. 2006;117:e257–261. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

