Engineered Hsp70 chaperones prevent Aβ42-induced memory impairments in a Drosophila model of Alzheimer's disease
- PMID: 29967544
- PMCID: PMC6028656
- DOI: 10.1038/s41598-018-28341-w
Engineered Hsp70 chaperones prevent Aβ42-induced memory impairments in a Drosophila model of Alzheimer's disease
Abstract
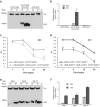
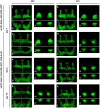
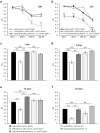
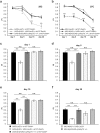
Proteinopathies constitute a group of diseases in which certain proteins are abnormally folded leading to aggregation and eventual cell failure. Most neurodegenerative diseases belong to protein misfolding disorders and, among them, Alzheimer's disease (AD) is the most prevalent. AD is characterized by accumulation of the amyloid-β42 (Aβ42) peptide in the extracellular space. Hence, we genetically engineered a molecular chaperone that was selectively delivered to this cellular location. It has been reported that the heat shock protein 70 (Hsp70) binds Aβ42 preventing self-aggregation. Here, we employed two isoforms of the Hsp70, cytosolic and extracellular, to evaluate their potential protective effect against the memory decline triggered by extracellular deposition of Aβ42. Both Hsp70 isoforms significantly improved memory performance of flies expressing Aβ42, irrespective of their age or the level of Aβ42 load. Using olfactory classical conditioning, we established a Drosophila model of AD based on Aβ42 neurotoxicity and monitored memory decline through aging. The onset of the memory impairment observed was proportional to the cumulative level of Aβ42 in the Drosophila brain. These data support the use of this Drosophila model of AD to further investigate molecules with a protective activity against Aβ42-induced memory loss, contributing to the development of palliative therapies for AD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Holdase activity of secreted Hsp70 masks amyloid-β42 neurotoxicity in Drosophila.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):E5212-21. doi: 10.1073/pnas.1608045113. Epub 2016 Aug 16. Proc Natl Acad Sci U S A. 2016. PMID: 27531960 Free PMC article.
-
Anti-Aβ single-chain variable fragment antibodies restore memory acquisition in a Drosophila model of Alzheimer's disease.Sci Rep. 2017 Sep 12;7(1):11268. doi: 10.1038/s41598-017-11594-2. Sci Rep. 2017. PMID: 28900185 Free PMC article.
-
secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids.Fly (Austin). 2017 Jul 3;11(3):179-184. doi: 10.1080/19336934.2017.1291104. Epub 2017 Feb 6. Fly (Austin). 2017. PMID: 28165856 Free PMC article.
-
Drosophila models of Alzheimer's amyloidosis: the challenge of dissecting the complex mechanisms of toxicity of amyloid-beta 42.J Alzheimers Dis. 2008 Dec;15(4):523-40. doi: 10.3233/jad-2008-15402. J Alzheimers Dis. 2008. PMID: 19096154 Review.
-
Chaperones-A New Class of Potential Therapeutic Targets in Alzheimer's Disease.Int J Mol Sci. 2024 Mar 17;25(6):3401. doi: 10.3390/ijms25063401. Int J Mol Sci. 2024. PMID: 38542375 Free PMC article. Review.
Cited by
-
Regulation of neuroimmune processes by damage- and resolution-associated molecular patterns.Neural Regen Res. 2021 Mar;16(3):423-429. doi: 10.4103/1673-5374.293134. Neural Regen Res. 2021. PMID: 32985460 Free PMC article. Review.
-
Hsp70 affects memory formation and behaviorally relevant gene expression in Drosophila melanogaster.Cell Stress Chaperones. 2021 May;26(3):575-594. doi: 10.1007/s12192-021-01203-7. Epub 2021 Apr 7. Cell Stress Chaperones. 2021. PMID: 33829398 Free PMC article.
-
Comparing In vitro Protein Aggregation Modelling Using Strategies Relevant to Neuropathologies.Cell Mol Neurobiol. 2025 Mar 13;45(1):24. doi: 10.1007/s10571-025-01539-z. Cell Mol Neurobiol. 2025. PMID: 40080205 Free PMC article.
-
Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease.Adv Exp Med Biol. 2020;1243:53-68. doi: 10.1007/978-3-030-40204-4_4. Adv Exp Med Biol. 2020. PMID: 32297211 Free PMC article. Review.
-
On the nature of the optimal form of the holdase-type chaperone stress response.FEBS Lett. 2020 Jan;594(1):43-66. doi: 10.1002/1873-3468.13580. Epub 2019 Sep 21. FEBS Lett. 2020. PMID: 31432502 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

