Impaired Neurofilament Integrity and Neuronal Morphology in Different Models of Focal Cerebral Ischemia and Human Stroke Tissue
- PMID: 29967576
- PMCID: PMC6015914
- DOI: 10.3389/fncel.2018.00161
Impaired Neurofilament Integrity and Neuronal Morphology in Different Models of Focal Cerebral Ischemia and Human Stroke Tissue
Abstract
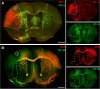
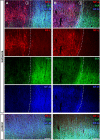
As part of the neuronal cytoskeleton, neurofilaments are involved in maintaining cellular integrity. In the setting of ischemic stroke, the affection of the neurofilament network is considered to mediate the transition towards long-lasting tissue damage. Although peripheral levels of distinct neurofilament subunits are shown to correlate with the clinically observed severity of cerebral ischemia, neurofilaments have so far not been considered for neuroprotective approaches. Therefore, the present study systematically addresses ischemia-induced alterations of the neurofilament light (NF-L), medium (NF-M), and heavy (NF-H) subunits as well as of α-internexin (INA). For this purpose, we applied a multi-parametric approach including immunofluorescence labeling, western blotting, qRT-PCR and electron microscopy. Analyses comprised ischemia-affected tissue from three stroke models of middle cerebral artery occlusion (MCAO), including approaches of filament-based MCAO in mice, thromboembolic MCAO in rats, and electrosurgical MCAO in sheep, as well as human autoptic stroke tissue. As indicated by altered immunosignals, impairment of neurofilament subunits was consistently observed throughout the applied stroke models and in human tissue. Thereby, altered NF-L immunoreactivity was also found to reach penumbral areas, while protein analysis revealed consistent reductions for NF-L and INA in the ischemia-affected neocortex in mice. At the mRNA level, the ischemic neocortex and striatum exhibited reduced expressions of NF-L- and NF-H-associated genes, whereas an upregulation for Ina appeared in the striatum. Further, multiple fluorescence labeling of neurofilament proteins revealed spheroid and bead-like structural alterations in human and rodent tissue, correlating with a cellular edema and lost cytoskeletal order at the ultrastructural level. Thus, the consistent ischemia-induced affection of neurofilament subunits in animals and human tissue, as well as the involvement of potentially salvageable tissue qualify neurofilaments as promising targets for neuroprotective strategies. During ischemia formation, such approaches may focus on the maintenance of neurofilament integrity, and appear applicable as co-treatment to modern recanalizing strategies.
Keywords: MCAO; axonal spheroids; cerebral ischemia; neurofilaments; stroke; α-internexin.
Figures






Similar articles
-
Simultaneous alterations of oligodendrocyte-specific CNP, astrocyte-specific AQP4 and neuronal NF-L demarcate ischemic tissue after experimental stroke in mice.Neurosci Lett. 2019 Oct 15;711:134405. doi: 10.1016/j.neulet.2019.134405. Epub 2019 Jul 30. Neurosci Lett. 2019. PMID: 31374325
-
Up-regulation of neurofilament light chains is associated with diminished immunoreactivities for MAP2 and tau after ischemic stroke in rodents and in a human case.J Chem Neuroanat. 2016 Dec;78:140-148. doi: 10.1016/j.jchemneu.2016.09.004. Epub 2016 Sep 16. J Chem Neuroanat. 2016. PMID: 27644143
-
The Cytoskeletal Elements MAP2 and NF-L Show Substantial Alterations in Different Stroke Models While Elevated Serum Levels Highlight Especially MAP2 as a Sensitive Biomarker in Stroke Patients.Mol Neurobiol. 2021 Aug;58(8):4051-4069. doi: 10.1007/s12035-021-02372-3. Epub 2021 May 1. Mol Neurobiol. 2021. PMID: 33931805 Free PMC article.
-
Neurofilaments and Neurofilament Proteins in Health and Disease.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a018309. doi: 10.1101/cshperspect.a018309. Cold Spring Harb Perspect Biol. 2017. PMID: 28373358 Free PMC article. Review.
-
Neurofilaments in health and disease.Prog Nucleic Acid Res Mol Biol. 1998;61:1-23. doi: 10.1016/s0079-6603(08)60823-5. Prog Nucleic Acid Res Mol Biol. 1998. PMID: 9752717 Review.
Cited by
-
Activation of neuronal Ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and axonal plasticity in mice.J Neurochem. 2021 May;157(4):1366-1376. doi: 10.1111/jnc.15195. Epub 2020 Oct 6. J Neurochem. 2021. PMID: 32964455 Free PMC article.
-
Increased Immunosignals of Collagen IV and Fibronectin Indicate Ischemic Consequences for the Neurovascular Matrix Adhesion Zone in Various Animal Models and Human Stroke Tissue.Front Physiol. 2020 Oct 26;11:575598. doi: 10.3389/fphys.2020.575598. eCollection 2020. Front Physiol. 2020. PMID: 33192578 Free PMC article.
-
Tau, Glial Fibrillary Acidic Protein, and Neurofilament Light Chain as Brain Protein Biomarkers in Cerebrospinal Fluid and Blood for Diagnosis of Neurobiological Diseases.Int J Mol Sci. 2024 Jun 7;25(12):6295. doi: 10.3390/ijms25126295. Int J Mol Sci. 2024. PMID: 38928000 Free PMC article. Review.
-
A Trem2R47H mouse model without cryptic splicing drives age- and disease-dependent tissue damage and synaptic loss in response to plaques.Mol Neurodegener. 2023 Feb 17;18(1):12. doi: 10.1186/s13024-023-00598-4. Mol Neurodegener. 2023. PMID: 36803190 Free PMC article.
-
Transcriptional Response and Morphological Features of the Neurovascular Unit and Associated Extracellular Matrix After Experimental Stroke in Mice.Mol Neurobiol. 2019 Nov;56(11):7631-7650. doi: 10.1007/s12035-019-1604-4. Epub 2019 May 14. Mol Neurobiol. 2019. PMID: 31089963 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

