Hodgkin Lymphoma-Derived Extracellular Vesicles Change the Secretome of Fibroblasts Toward a CAF Phenotype
- PMID: 29967610
- PMCID: PMC6015880
- DOI: 10.3389/fimmu.2018.01358
Hodgkin Lymphoma-Derived Extracellular Vesicles Change the Secretome of Fibroblasts Toward a CAF Phenotype
Abstract
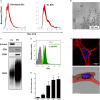
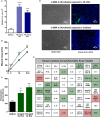
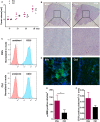
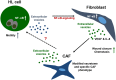
Secretion of extracellular vesicles (EVs) is a ubiquitous mechanism of intercellular communication based on the exchange of effector molecules, such as growth factors, cytokines, and nucleic acids. Recent studies identified tumor-derived EVs as central players in tumor progression and the establishment of the tumor microenvironment (TME). However, studies on EVs from classical Hodgkin lymphoma (cHL) are limited. The growth of malignant Hodgkin and Reed-Sternberg (HRS) cells depends on the TME, which is actively shaped by a complex interaction of HRS cells and stromal cells, such as fibroblasts and immune cells. HRS cells secrete cytokines and angiogenic factors thus recruiting and inducing the proliferation of surrounding cells to finally deploy an immunosuppressive TME. In this study, we aimed to investigate the role of tumor cell-derived EVs within this complex scenario. We observed that EVs collected from Hodgkin lymphoma (HL) cells were internalized by fibroblasts and triggered their migration capacity. EV-treated fibroblasts were characterized by an inflammatory phenotype and an upregulation of alpha-smooth muscle actin (α-SMA), a marker of cancer-associated fibroblasts. Analysis of the secretome of EV-treated fibroblast revealed an enhanced release of pro-inflammatory cytokines (e.g., IL-1α, IL-6, and TNF-α), growth factors (G-CSF and GM-CSF), and pro-angiogenic factors such as VEGF. These soluble factors are known to promote HL progression. In line, ingenuity pathway analysis identified inflammatory pathways, including TNF-α/NF-κB-signaling, as key factors directing the EV-dependent phenotype changes in fibroblasts. Confirming the in vitro data, we demonstrated that EVs promote α-SMA expression in fibroblasts and the expression of proangiogenic factors using a xenograft HL model. Collectively, we demonstrate that HL EVs alter the phenotype of fibroblasts to support tumor growth, and thus shed light on the role of EVs for the establishment of the tumor-promoting TME in HL.
Keywords: Hodgkin lymphoma; NF-κB-signaling; cancer-associated fibroblasts; extracellular vesicles; tumor microenvironment.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

