Establishing Analytical Performance Criteria for the Global Reconnaissance of Antibiotics and Other Pharmaceutical Residues in the Aquatic Environment Using Liquid Chromatography-Tandem Mass Spectrometry
- PMID: 29967712
- PMCID: PMC6008649
- DOI: 10.1155/2018/7019204
Establishing Analytical Performance Criteria for the Global Reconnaissance of Antibiotics and Other Pharmaceutical Residues in the Aquatic Environment Using Liquid Chromatography-Tandem Mass Spectrometry
Abstract
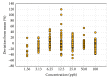
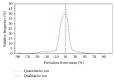
The occurrence of antibiotics in the environment from discharges of wastewater treatment plants (WWTPs) and from the land application of antibiotic-laden manure from animal agriculture is a critical global issue because these residues have been associated with the increased emergence of antibiotic resistance in the environment. In addition, other classes of pharmaceuticals and personal care products (PPCPs) have been found in effluents of municipal WWTPs, many of which persist in the receiving environments. Analysis of antibiotics by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in samples from different countries presents unique challenges that should be considered, from ion suppression due to matrix effects, to lack of available stable isotopically labeled standards for accurate quantification. Understanding the caveats of LC-MS/MS is important for assessing samples with varying matrix complexity. Ion ratios between quantifying and qualifying ions have been used for quality assurance purposes; however, there is limited information regarding the significance of setting criteria for acceptable variabilities in their values in the literature. Upon investigation of 30 pharmaceuticals in WWTP influent and effluent samples, and in receiving surface water samples downstream and upstream of the WWTP, it was found that ion ratios have higher variabilities at lower concentrations in highly complex matrices, and the extent of variability may be exacerbated by the physicochemical properties of the analytes. In setting the acceptable ion ratio criterion, the overall mean, which was obtained by taking the average of the ion ratios at all concentrations (1.56 to 100 ppb), was used. Then, for many of the target analytes included in this study, the tolerance range was set at 40% for WWTP influent samples and 30% for WWTP effluent, upstream, and downstream samples. A separate tolerance range of 80% was set for tetracyclines and quinolones, which showed higher variations in the ion ratios compared to the other analytes.
Figures



Similar articles
-
Towards a harmonized method for the global reconnaissance of multi-class antimicrobials and other pharmaceuticals in wastewater and receiving surface waters.Environ Int. 2019 Mar;124:361-369. doi: 10.1016/j.envint.2019.01.025. Epub 2019 Jan 17. Environ Int. 2019. PMID: 30660849
-
Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada.Sci Total Environ. 2006 Aug 31;367(2-3):544-58. doi: 10.1016/j.scitotenv.2006.03.021. Epub 2006 May 12. Sci Total Environ. 2006. PMID: 16697441
-
Environmental exposure of pharmaceuticals and musk fragrances in the Somes River before and after upgrading the municipal wastewater treatment plant Cluj-Napoca, Romania.Environ Sci Pollut Res Int. 2009 Aug;16 Suppl 1:S46-54. doi: 10.1007/s11356-008-0047-7. Epub 2008 Oct 30. Environ Sci Pollut Res Int. 2009. PMID: 18972147
-
Determination of estrogenic compounds in wastewater using liquid chromatography-tandem mass spectrometry with electrospray and atmospheric pressure photoionization following desalting extraction.Chemosphere. 2009 Jan;74(4):508-14. doi: 10.1016/j.chemosphere.2008.09.089. Epub 2008 Nov 8. Chemosphere. 2009. PMID: 18996561
-
Trace analysis and occurrence of anhydroerythromycin and tylosin in influent and effluent wastewater by liquid chromatography combined with electrospray tandem mass spectrometry.Anal Bioanal Chem. 2006 Jun;385(3):623-36. doi: 10.1007/s00216-006-0416-3. Epub 2006 May 3. Anal Bioanal Chem. 2006. PMID: 16715282
Cited by
-
Robust UPLC-MS/MS Method With Acetonitrile for Precise Intracellular Quantification of Tacrolimus in PBMCs: A Step Toward Clinical Integration.Clin Transl Sci. 2025 Apr;18(4):e70210. doi: 10.1111/cts.70210. Clin Transl Sci. 2025. PMID: 40145774 Free PMC article.
-
Determination of Pharmaceutical Residues by UPLC-MS/MS Method: Validation and Application on Surface Water and Hospital Wastewater.J Anal Methods Chem. 2021 Jan 8;2021:6628285. doi: 10.1155/2021/6628285. eCollection 2021. J Anal Methods Chem. 2021. PMID: 33505763 Free PMC article.
-
A machine learning framework to predict PPCP removal through various wastewater and water reuse treatment trains.Environ Sci (Camb). 2024 Dec 19;11(2):481-493. doi: 10.1039/d4ew00892h. eCollection 2025 Jan 30. Environ Sci (Camb). 2024. PMID: 39758590 Free PMC article.
-
Repellent active ingredients encapsulated in polymeric nanoparticles: potential alternative formulations to control arboviruses.J Nanobiotechnology. 2022 Dec 10;20(1):520. doi: 10.1186/s12951-022-01729-7. J Nanobiotechnology. 2022. PMID: 36496396 Free PMC article.
References
-
- Senta I., Krizman-Matasic I., Terzic S., Ahel M. Comprehensive determination of macrolide antibiotics, their synthesis intermediates and transformation products in wastewater effluents and ambient waters by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A. 2017;1509:60–68. doi: 10.1016/j.chroma.2017.06.005. - DOI - PubMed
-
- Senta I., Terzić S., Ahel M. Simultaneous determination of sulfonamides, fluoroquinolones, macrolides and trimethoprim in wastewater and river water by LC-tandem-MS. Chromatographia. 2008;68(9-10):747–758. doi: 10.1365/s10337-008-0780-6. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

