Microbiota Composition and the Integration of Exogenous and Endogenous Signals in Reactive Nasal Inflammation
- PMID: 29967798
- PMCID: PMC6008798
- DOI: 10.1155/2018/2724951
Microbiota Composition and the Integration of Exogenous and Endogenous Signals in Reactive Nasal Inflammation
Abstract
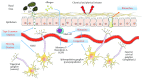
The prevalence of reactive nasal inflammatory conditions, for example, allergic rhinitis and chronic rhinosinusitis, is steadily increasing in parallel with significant environmental changes worldwide. Allergens and as yet undefined environmental agents may trigger these conditions via the involvement of host intrinsic factors, including the innate and adaptive immune system, the nasal epithelium, and the nasal nervous system. The critical role of the nasal microbiota in coordinating these components has emerged in recent studies documenting a significant association between microbial composition and the onset and progression of allergic or nonallergic inflammation. It is now clear that the local microbiota is a major player in the development of the mucosa-associated lymphoid tissue and in the regulation of such adaptive responses as IgA production and the function of effector and regulatory T cells. Microbial components also play a major role in the regulation of epithelial barrier functions, including mucus production and the control of paracellular transport across tight junctions. Bacterial components, including lipopolysaccharide, have also been shown to induce or amplify neuroinflammatory responses by engaging specific nociceptors. Finally, bacterial products may promote tissue remodeling processes, including nasal polyp formation, by interacting with formyl peptide receptors and inducing the expression of angiogenic factors and matrix-degrading enzymes.
Figures

References
-
- Bodino C., Jankowski R., Grignon B., Jimenez-Chobillon A., Braun M. Surgical anatomy of the turbinal wall of the ethmoidal labyrinth. Rhinology. 2004;42(2):73–80. - PubMed
-
- D’Amato G., Holgate S. T., Pawankar R., et al. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organization Journal. 2015;8(1):1–52. doi: 10.1186/s40413-015-0073-0. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

