Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion
- PMID: 29968069
- PMCID: PMC6086790
- DOI: 10.1007/s10815-018-1231-4
Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion
Abstract
Purpose: To study the regulation and functions of oviductal glycoprotein 1 (OVGP1) in endometrial epithelial cells.
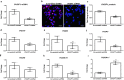
Methods: Expression of OVGP1 in mouse endometrium during pregnancy and in the endometrial epithelial cell line (Ishikawa) was studied by immunofluorescence, Western blotting, and RT-PCR. Regulation of OVGP1 in response to ovarian steroids and human chorionic gonadotropin (hCG) was studied by real-time RT-PCR. OVGP1 expression was knockdown in Ishikawa cells by shRNA, and expression of receptivity associated genes was studied by real-time RT-PCR. Adhesion of trophoblast cell line (JAr) was studied by in vitro adhesion assays.
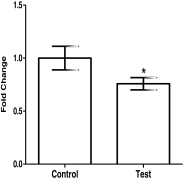
Results: OVGP1 was localized exclusively in the luminal epithelial cells of mouse endometrium at the time of embryo implantation. Along with estrogen and progesterone, hCG induced the expression of OVGP1 in Ishikawa cells. Knockdown of OVGP1 in Ishikawa cells reduced mRNA expression of ITGAV, ITGB3, ITGA5, HOXA10, LIF, and IL15; it increased the expression of HOXA11, MMP9, TIMP1, and TIMP3. Supernatants derived from OVGP1 knockdown Ishikawa cells reduced the adhesiveness of JAr cells in vitro. Expression of OVGP1 mRNA was found to be significantly lowered in the endometrium of women with recurrent implantation failure.
Conclusion: OVGP1 is specifically induced in the luminal epithelium at the time of embryo implantation where it regulates receptivity-related genes and aids in trophoblast adhesion.
Keywords: Endometrium; Glycoprotein; Implantation; Oviductal glycoprotein 1; Receptivity; Trophoblast.
Conflict of interest statement
The study was approved by the Institutional Animal Ethics Committee (IAEC) of the National Institute for Research in Reproductive (NIRRH).
The authors declare that they have no conflict of interests.
Figures




References
-
- Modi DN, Bhartiya P. Physiology of embryo-endometrial cross talk. Biomed Res J. 2015;2:83–104.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

