Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling
- PMID: 29968249
- PMCID: PMC6109221
- DOI: 10.1111/bph.14435
Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling
Abstract
Background and purpose: Autism spectrum disorder (ASD) is more commonly diagnosed in males than in females. Prenatal exposure to the antiepileptic drug valproic acid (VPA) is an environmental risk factor of ASD. Male rats prenatally exposed to VPA show socio-emotional autistic-like dysfunctions that have been related to changes in the activity of the endocannabinoid anandamide. Here, we have investigated if prenatal VPA induced sex-specific autistic endophenotypes involving anandamide signalling.
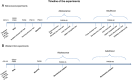
Experimental approach: We studied sex-specific differences in the ASD-like socio-emotional, cognitive and repetitive symptoms displayed during development of Wistar rats of both sexes, prenatally exposed to VPA. The involvement of anandamide was followed by Western blotting of cannabinoid CB1 receptors and by inhibiting its metabolism.
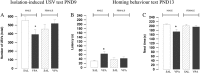
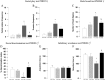
Key results: Female rats were less vulnerable to the deleterious effects of prenatal VPA exposure on social communication, emotional reactivity and cognitive performance than male rats. Conversely, as observed in male rats, prenatal VPA exposure induced selective deficits in social play behaviour and stereotypies in the female rat offspring. At the neurochemical level, prenatal VPA exposure altered phosphorylation of CB1 receptors in a sex-specific, age-specific and tissue-specific manner. Enhancing anandamide signalling through inhibition of its degradation reversed the behavioural deficits displayed by VPA-exposed animals of both sexes.
Conclusions and implications: These findings highlight sexually dimorphic consequences of prenatal VPA exposure that may be related to sex-specific effects of VPA on endocannabinoid neurotransmission in the course of development and introduce a new therapeutic target for reversing autistic-like symptoms in both sexes.
© 2018 The British Pharmacological Society.
Figures



References
-
- American Psychiatric Association (2013). Diagnostic and Statistic Manual of Mental Disorders: DMS‐5, 5th edn: Washington, DC, USA.
-
- Banach R, Thompson A, Szatmari P, Goldberg J, Tuff L, Zwaigenbaum L et al (2009). Brief report: relationship between non‐verbal IQ and gender in autism. J Autism Dev Disord 39: 188–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

