Paliperidone palmitate once-every-3-months in adults with early illness schizophrenia
- PMID: 29968279
- PMCID: PMC6585630
- DOI: 10.1111/eip.12685
Paliperidone palmitate once-every-3-months in adults with early illness schizophrenia
Abstract
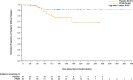
Aim: This post hoc analysis of a double-blind (DB), randomized, placebo-controlled, relapse-prevention study evaluated the effects of paliperidone palmitate once-every-3-months (PP3M) in a subpopulation of adults with early illness schizophrenia (duration ≤5 years) from a clinical trial.
Methods: Patients received either PP3M or placebo every 3 months in the DB phase. The primary efficacy variable was time from randomization to first relapse. Symptom severity, patient functioning, and safety were also assessed.
Results: A total of 119 patients who entered the DB phase met the criteria for early illness schizophrenia (PP3M, n = 62; placebo, n = 57). PP3M significantly delayed time to relapse vs placebo (P = .035; hazard ratio, 3.08; 95% CI, 1.08-8.80). Symptomatic control and patient functioning were maintained in the PP3M group but significantly worsened in the placebo group. There were no unexpected tolerability findings.
Conclusions: PP3M reduced relapse risk and maintained symptomatic and functional improvements compared with placebo in patients with early illness schizophrenia.
Keywords: early illness; paliperidone palmitate; recently diagnosed; relapse; schizophrenia.
© 2018 The Authors Early Intervention in Psychiatry Published by John Wiley & Sons Australia, Ltd.
Figures
References
-
- Alphs, L. , Bossie, C. , Mao, L. , Lee, E. , & Starr, H. L. (2015). Treatment effect with paliperidone palmitate compared with oral antipsychotics in patients with recent‐onset versus more chronic schizophrenia and a history of criminal justice system involvement. Early Intervention in Psychiatry, 12(1), 55–65. - PMC - PubMed
-
- Altamura, A. C. , Aguglia, E. , Bassi, M. , Bogetto, F. , Cappellari, L. , De Giorgi, S. , … Girardi, P. (2012). Rethinking the role of long‐acting atypical antipsychotics in the community setting. International Clinical Psychopharmacology, 27(6), 336–349. - PubMed
-
- Berwaerts, J. , Liu, Y. , Gopal, S. , Nuamah, I. , Xu, H. , Savitz, A. , … Hough, D. W. (2015). Efficacy and safety of the 3‐month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry, 72(8), 830–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

