Chronic exercise impairs nitric oxide pathway in rabbit carotid and femoral arteries
- PMID: 29968308
- PMCID: PMC6138298
- DOI: 10.1113/JP275611
Chronic exercise impairs nitric oxide pathway in rabbit carotid and femoral arteries
Abstract
Key points: Some of the beneficial effects of exercise in preventing vascular related diseases are mediated by the enhancement of endothelial function where the role of nitric oxide (NO) is well documented, although the relevance of calcium activated potassium channels is not fully understood. The impact of oxidative stress induced by training on endothelial function remains to be clarified. By evaluating different endothelial vasodilator pathways on two vascular beds in a rabbit model of chronic exercise, we found a decreased NO bioavailability and endothelial nitric oxide synthase expression in both carotid and femoral arteries. Physical training induced carotid endothelial dysfunction as a result of an increase in oxidative stress and a reduction in superoxide dismutase expression. In the femoral artery, the lower production of NO was counteracted by an increased participation of large conductance calcium activated potassium channels, preventing endothelial dysfunction.
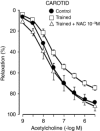
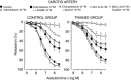
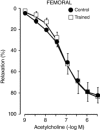
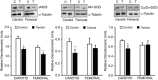
Abstract: The present study aimed to evaluate the effects of chronic exercise on vasodilator response in two different arteries. Rings of carotid and femoral arteries from control and trained rabbits were suspended in organ baths for isometric recording of tension. Endothelial nitric oxide synthase (eNOS), Cu/Zn and Mn-superoxide dismutase (SOD), and large conductance calcium activated potassium (BKCa) channel protein expression were measured by western blotting. In the carotid artery, training reduced the relaxation to ACh (10-9 to 3 × 10-6 m) that was reversed by N-acetylcysteine (10-3 m). l-NAME (10-4 m) reduced the relaxation to ACh in both groups, although the effect was lower in the trained group (in mean ± SEM, 39 ± 2% vs. 28 ± 3%). Physical training did not modify the relaxation to ACh in femoral arteries, although the response to l-NAME was lower in the trained group (in mean ± SEM, 41 ± 5% vs. 17 ± 2%). Charybdotoxin (10-7 m) plus apamin (10-6 m) further reduced the maximal relaxation to ACh only in the trained group. The remaining relaxation in both carotid and femoral arteries was abolished by KCl (2 × 10-2 m) and BaCl2 (3 × 10-6 m) plus ouabain (10-4 m) in both groups. Physical training decreased eNOS expression in both carotid and femoral arteries and Cu/Zn and Mn-SOD expression only in the carotid artery. BKCa channels were overexpressed in the trained group in the femoral artery. In conclusion, chronic exercise induces endothelial dysfunction in the carotid artery as a result of oxidative stress. In the femoral artery, it modifies the vasodilator pathways, enhancing the participation of BKCa channels, thus compensating for the impairment of NO-mediated vasodilatation.
Keywords: Nitric oxide; calcium activated potassium channels; chronic exercise; endothelial dysfunction; oxidative stress.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures



References
-
- Abdulnour R‐EE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE, Kayyali US, Garcia JGN, Tuder RM & Hassoun PM (2006). Mechanical stress activates xanthine oxidoreductase through MAP kinase‐dependent pathways. Am J Physiol Lung Cell Mol Physiol 291, L345–L353. - PubMed
-
- Bergholm R, Mäkimattila S, Valkonen M, Liu ML, Lahdenperä S, Taskinen MR, Sovijärvi A, Malmberg P & Yki‐Järvinen H (1999). Intense physical training decreases circulating antioxidants and endothelium‐dependent vasodilatation in vivo. Atherosclerosis 145, 341–349. - PubMed
-
- Black JM, Stöhr EJ, Shave R & Esformes JI (2016). Influence of exercise training mode on arterial diameter: a systematic review and meta‐analysis. J Sci Med Sport 19, 74–80. - PubMed
-
- Chen Hi H, Chiang I‐P & Jen CJ (1996). Exercise training increases acetylcholine‐stimulated endothelium‐derived nitric oxide release in spontaneously hypertensive rats. J Biomed Sci 3, 454–460. - PubMed
-
- Chen HI & Li HT (1993). Physical conditioning can modulate endothelium‐dependent vasorelaxation in rabbits. Arterioscler Thromb J Vasc Biol 13, 852–856. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

