Activation of sterol regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma
- PMID: 29968360
- PMCID: PMC6175466
- DOI: 10.1002/path.5130
Activation of sterol regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma
Abstract
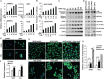
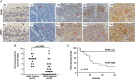
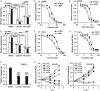
Nasopharyngeal carcinoma (NPC) is closely associated with Epstein-Barr virus (EBV) infection. The EBV-encoded latent membrane protein 1 (LMP1), which is commonly expressed in NPC, engages multiple signaling pathways that promote cell growth, transformation, and metabolic reprogramming. Here, we report a novel function of LMP1 in promoting de novo lipogenesis. LMP1 increases the expression, maturation and activation of sterol regulatory element-binding protein 1 (SREBP1), a master regulator of lipogenesis, and its downstream target fatty acid synthase (FASN). LMP1 also induces de novo lipid synthesis and lipid droplet formation. In contrast, small interfering RNA (siRNA) knockdown of LMP1 in EBV-infected epithelial cells diminished SREBP1 activation and lipid biosynthesis. Furthermore, inhibition of the mammalian target of rapamycin (mTOR) pathway, through the use of either mTOR inhibitors or siRNAs, significantly reduced LMP1-mediated SREBP1 activity and lipogenesis, indicating that LMP1 activation of the mTOR pathway is required for SREBP1-mediated lipogenesis. In primary NPC tumors, FASN overexpression is common, with high levels correlating significantly with LMP1 expression. Moreover, elevated FASN expression was associated with aggressive disease and poor survival in NPC patients. Luteolin and fatostatin, two inhibitors of lipogenesis, suppressed lipogenesis and proliferation of nasopharyngeal epithelial cells, effects that were more profound in cells expressing LMP1. Luteolin and fatostatin also dramatically inhibited NPC tumor growth in vitro and in vivo. Our findings demonstrate that LMP1 activation of SREBP1-mediated lipogenesis promotes tumor cell growth and is involved in EBV-driven NPC pathogenesis. Our results also reveal the therapeutic potential of utilizing lipogenesis inhibitors in the treatment of locally advanced or metastatic NPC. © 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: Epstein-Barr virus; LMP1; SREBP1; lipogenesis; nasopharyngeal carcinoma.
© 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures



References
-
- Dawson CW, Port RJ, Young LS. The role of the EBV‐encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 2012; 22 : 144–153. - PubMed
-
- Lo AK, Lo KW, Ko CW, et al Inhibition of the LKB1–AMPK pathway by the Epstein–Barr virus‐encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol 2013; 230 : 336–346. - PubMed
-
- Lo AK, Dawson CW, Young LS, et al Activation of the FGFR1 signalling pathway by the Epstein–Barr virus‐encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J Pathol 2015; 237 : 238–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

