Hemochromatosis (HFE) Gene Variants Are Associated with Increased Mitochondrial DNA Levels During HIV-1 Infection and Antiretroviral Therapy
- PMID: 29968489
- PMCID: PMC6421985
- DOI: 10.1089/AID.2018.0025
Hemochromatosis (HFE) Gene Variants Are Associated with Increased Mitochondrial DNA Levels During HIV-1 Infection and Antiretroviral Therapy
Abstract
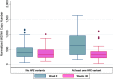
Some HIV-associated complications involve mitochondrial dysfunction and may be less common in individuals with iron-loading HFE (hemochromatosis gene) variants. We evaluated HFE 845A and 187G alleles in relation to mitochondrial DNA (mtDNA) levels in peripheral blood mononuclear cells from 85 individuals with HIV infection on uninterrupted antiretroviral therapy (ART) for 15 or more consecutive weeks. Carriers of HFE gene variants (N = 24) had significantly higher mtDNA levels than noncarriers (N = 61), after adjusting for age, race, sex, and type of ART [adjusted β-coefficient 297, p-value < .001 for at least one HFE variant], but mtDNA declined among all individuals on study during 48 weeks on ART. Increased cellular mtDNA content may represent a compensatory response to mitochondrial stress that is influenced by iron-loading HFE variants.
Keywords: ART; HFE; allele; mtDNA.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Gardner K, Hall PA, Chinnery PF, Payne BA: HIV treatment and associated mitochondrial pathology: Review of 25 years of in vitro, animal, and human studies. Toxicol Pathol 2014;42:811–822 - PubMed
-
- Lewis W, Day BJ, Copeland WC: Mitochondrial toxicity of NRTI antiviral drugs: An integrated cellular perspective. Nat Rev Drug Discov 2003;2:812–822 - PubMed
-
- Apostolova N, Blas-Garcia A, Esplugues JV: Mitochondrial toxicity in HAART: An overview of in vitro evidence. Curr Pharm Des 2011;17:2130–2144 - PubMed
-
- Dagan T, Sable C, Bray J, Gerschenson M: Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: What is the evidence? Mitochondrion 2002;1:397–412 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI077505/AI/NIAID NIH HHS/United States
- U01 AI027664/AI/NIAID NIH HHS/United States
- R01 NS044807/NS/NINDS NIH HHS/United States
- U01 AI025903/AI/NIAID NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- P20 RR016467/RR/NCRR NIH HHS/United States
- R01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI027668/AI/NIAID NIH HHS/United States
- U01 AI027670/AI/NIAID NIH HHS/United States
- R21 NS059330/NS/NINDS NIH HHS/United States
- R21 HL087726/HL/NHLBI NIH HHS/United States
- U54 MD007601/MD/NIMHD NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- U01 AI046386/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- U01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- K24 NS002253/NS/NINDS NIH HHS/United States
- P20 GM113134/GM/NIGMS NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI032770/AI/NIAID NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- U01 AI027658/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical