Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates
- PMID: 29968775
- PMCID: PMC6030104
- DOI: 10.1038/s41598-018-28296-y
Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates
Abstract
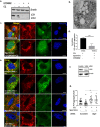
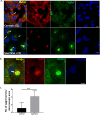
Autolysosomal dysfunction and unstable microtubules are hallmarks of chronic neurodegenerative diseases associated with misfolded proteins. Investigation of impaired protein quality control and clearance systems could therefore provide an important avenue for intervention. To investigate this we have used a highly controlled model for protein aggregation, an in vitro prion system. Here we report that prion aggregates traffic via autolysosomes in the cytoplasm. Treatment with the natural polyamine spermine clears aggregates by enhancing autolysosomal flux. We demonstrated this by blocking the formation of mature autophagosomes resulting in accumulation of prion aggregates in the cytoplasm. Further we investigated the mechanism of spermine's mode of action and we demonstrate that spermine increases the acetylation of microtubules, which is known to facilitate retrograde transport of autophagosomes from the cellular periphery to lysosomes located near the nucleus. We further report that spermine facilitates selective autophagic degradation of prion aggregates by binding to microtubule protein Tubb6. This is the first report in which spermine and the pathways regulated by it are applied as a novel approach towards clearance of misfolded prion protein and we suggest that this may have important implication for the broader family of protein misfolding diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases.J Cell Biol. 2017 Sep 4;216(9):2633-2644. doi: 10.1083/jcb.201701047. Epub 2017 Jul 19. J Cell Biol. 2017. PMID: 28724527 Free PMC article. Review.
-
S6K1 Is Indispensible for Stress-Induced Microtubule Acetylation and Autophagic Flux.Cells. 2021 Apr 17;10(4):929. doi: 10.3390/cells10040929. Cells. 2021. PMID: 33920542 Free PMC article.
-
Pharmacological activation of autophagy favors the clearing of intracellular aggregates of misfolded prion protein peptide to prevent neuronal death.Cell Death Dis. 2018 Feb 7;9(2):166. doi: 10.1038/s41419-017-0252-8. Cell Death Dis. 2018. PMID: 29416016 Free PMC article.
-
Rotenone Induces the Formation of 4-Hydroxynonenal Aggresomes. Role of ROS-Mediated Tubulin Hyperacetylation and Autophagic Flux Disruption.Mol Neurobiol. 2016 Nov;53(9):6194-6208. doi: 10.1007/s12035-015-9509-3. Epub 2015 Nov 12. Mol Neurobiol. 2016. PMID: 26558631
-
Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies.Exp Mol Med. 2015 Mar 13;47(3):e147. doi: 10.1038/emm.2014.117. Exp Mol Med. 2015. PMID: 25766616 Free PMC article. Review.
Cited by
-
Acetylation modification in the regulation of macroautophagy.Adv Biotechnol (Singap). 2024 Jun 7;2(2):19. doi: 10.1007/s44307-024-00027-7. Adv Biotechnol (Singap). 2024. PMID: 39883319 Free PMC article. Review.
-
An Update on Autophagy in Prion Diseases.Front Bioeng Biotechnol. 2020 Aug 27;8:975. doi: 10.3389/fbioe.2020.00975. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984276 Free PMC article. Review.
-
Loss of Deacetylation Enzymes Hdac6 and Sirt2 Promotes Acetylation of Cytoplasmic Tubulin, but Suppresses Axonemal Acetylation in Zebrafish Cilia.Front Cell Dev Biol. 2021 Jun 28;9:676214. doi: 10.3389/fcell.2021.676214. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34268305 Free PMC article.
-
Altered brain arginine metabolism in a mouse model of tauopathy.Amino Acids. 2019 Mar;51(3):513-528. doi: 10.1007/s00726-018-02687-x. Epub 2019 Jan 2. Amino Acids. 2019. PMID: 30604097 Free PMC article.
-
Full restoration of specific infectivity and strain properties from pure mammalian prion protein.PLoS Pathog. 2019 Mar 25;15(3):e1007662. doi: 10.1371/journal.ppat.1007662. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30908557 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/D/20251968/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004332/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BBS/E/D/20002173/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT104915MA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

