Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence
- PMID: 29968804
- PMCID: PMC6030087
- DOI: 10.1038/s41598-018-28443-5
Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence
Abstract
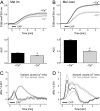
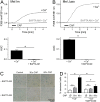
Cold atmospheric plasma (CAP) is a promising approach in anti-cancer therapy, eliminating cancer cells with high selectivity. However, the molecular mechanisms of CAP action are poorly understood. In this study, we investigated CAP effects on calcium homeostasis in melanoma cells. We observed increased cytoplasmic calcium after CAP treatment, which also occurred in the absence of extracellular calcium, indicating the majority of the calcium increase originates from intracellular stores. Application of previously CAP-exposed extracellular solutions also induced cytoplasmic calcium elevations. A substantial fraction of this effect remained when the application was delayed for one hour, indicating the chemical stability of the activating agent(s). Addition of ryanodine and cyclosporin A indicate the involvement of the endoplasmatic reticulum and the mitochondria. Inhibition of the cytoplasmic calcium elevation by the intracellular chelator BAPTA blocked CAP-induced senescence. This finding helps to understand the molecular influence and the mode of action of CAP on tumor cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Weltmann K-D, von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Phys. Control. Fusion. 2017;59:14031. doi: 10.1088/0741-3335/59/1/014031. - DOI
-
- Heinlin J, et al. Plasma medicine: possible applications in dermatology. JDDG - J. Ger. Soc. Dermatology. 2010;8:968–977. - PubMed
-
- Fridman G, et al. Applied plasma medicine. Plasma Process. Polym. 2008;5:503–533. doi: 10.1002/ppap.200700154. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

