Mechanochemical Effects on Extracellular Signal-Regulated Kinase Dynamics in Stem Cell Differentiation
- PMID: 29969368
- PMCID: PMC6080114
- DOI: 10.1089/ten.tea.2017.0365
Mechanochemical Effects on Extracellular Signal-Regulated Kinase Dynamics in Stem Cell Differentiation
Abstract
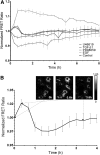
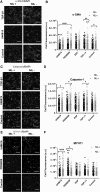
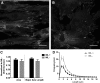
Understanding how key signaling molecules are coregulated by biochemical agents and physical stimuli during stem cell differentiation is critical but often lacking. Due to the important role of extracellular signal-regulated kinase (ERK), this study has examined its temporal dynamics to determine the coregulation of mechanochemical cues on ERK phosphorylation for smooth muscle cell (SMC) differentiation. To assess ERK1/2 activity, a fluorescence resonance energy transfer-based biosensor was transfected into mesenchymal stem cells. The influences of nanopatterned substrates, growth factors, and drugs on ERK activities were related to their effects on SMC differentiation. Results revealed that nanopatterned substrates significantly increased ERK activity in cells, overriding ERK response from administered biochemical factors. The nanopatterned substrates reduced expression of SMC markers after a 48-h biochemical treatment, except for the combination with ERK inhibitor PD98059 treatment, which enhanced expression of mature SMC marker MYH11. Immunofluorescent staining for focal adhesion proteins, vinculin and zyxin, indicated no significant differences in vinculin cluster distribution or dimension, while the location of zyxin changed from adhesion sites of cell periphery on nonpatterned substrate to actin filaments on nanopatterned substrate. The zyxin-reinforced stress fibers likely enhanced the cytoskeletal tension to increase ERK dynamics. Collectively, results suggest that physical stimuli play a dominating role in initial ERK signaling and early-stage differentiation through focal adhesion changes, and the capability of monitoring signaling events in real time could be exploited to guide the engineering of cell microenvironment.
Keywords: ERK phosphorylation; FRET; mechanochemical stimuli; nanopattern.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Reilly G.C., and Engler A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43, 55, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

