Peyer's patches-derived CD11b+ B cells recruit regulatory T cells through CXCL9 in dextran sulphate sodium-induced colitis
- PMID: 29969845
- PMCID: PMC6187209
- DOI: 10.1111/imm.12977
Peyer's patches-derived CD11b+ B cells recruit regulatory T cells through CXCL9 in dextran sulphate sodium-induced colitis
Abstract
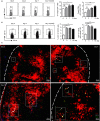
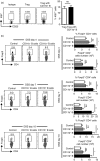
Regulatory T (Treg) cells play an essential role in the maintenance of intestinal homeostasis. In Peyer's patches (PPs), which comprise the most important IgA induction site in the gut-associated lymphoid tissue, Treg cells promote IgA isotype switching. However, the mechanisms underlying their entry into PPs and isotype switching facilitation in activated B cells remain unknown. This study, based on the dextran sulphate sodium (DSS)-induced colitis model, revealed that Treg cells are significantly increased in PPs, along with CD11b+ B-cell induction. Immunofluorescence staining showed that infiltrated Treg cells were located around CD11b+ B cells and produced transforming growth factor-β, thereby inducing IgA+ B cells. Furthermore, in vivo and in vitro studies revealed that CD11b+ B cells in PPs had the capacity to recruit Treg cells into PPs rather than promoting their proliferation. Finally, we found that Treg cell recruitment was mediated by the chemokine CXCL9 derived from CD11b+ B cells in PPs. These findings demonstrate that CD11b+ B cells induced in PPs during colitis actively recruit Treg cells to accomplish IgA isotype switch in a CXCL9-dependent manner.
Keywords: CD11b+ B cells; colitis; regulatory T cells.
© 2018 John Wiley & Sons Ltd.
Figures


References
-
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012; 30:221–41. - PubMed
-
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 2008; 224:201–14. - PubMed
-
- Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y et al Foxp3+ T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014; 41:152–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

