Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment
- PMID: 29970037
- PMCID: PMC6029116
- DOI: 10.1186/s12879-018-3192-8
Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment
Abstract
Background: Tuberculosis (TB) and chronic Hepatitis B virus (HBV) infection are common in China. Fist-line anti-TB medications often produce drug-induced liver injury (DILI). This study sought to investigate whether TB patients with chronic HBV co-infection are more susceptible to liver failure and poor outcomes during anti-TB treatment.
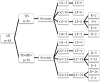
Methods: Eighty-four TB patients developed DILI during anti-TB treatment and were enrolled, including 58 with chronic HBV co-infection (TB-HBV group) and 26 with TB mono-infection (TB group). Clinical data and demographic characteristics were reviewed. The severity of DILI and incidences of liver failure and death were compared. Risk factors of clinical outcomes were defined.
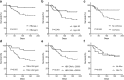
Results: The patterns of DILI were similar in both groups. Compared with patients in the TB group, patients in the TB-HBV group who did not receive anti-HBV therapy before anti-TB treatment were more susceptible to Grade-4 severity of DILI (36.2% vs. 7.7%, P = 0.005), liver failure (67.2% vs. 38.5%, P = 0.013) and poor outcomes (37.9% vs. 7.7%, P = 0.005). Age > 50 years (48.1% vs. 22.6%, P = 0.049), cirrhosis (50.0% vs. 15.4%, P = 0.046) and total bilirubin > 20 mg/dl (51.6% vs. 14.8%, P = 0.005) were independent risk factors for the rate of death in the TB-HBV group, and HBV DNA > 20,000 IU/ml had borderline significance (44.1% vs. 20.8%, P = 0.081). In the TB-HBV group, nucleos(t)ide analogues as rescue therapy were not able to reduce short-term death (33.3% vs. 36.8%, P = 0.659) once liver failure had occurred.
Conclusions: Patients on anti-TB therapy with chronic HBV co-infection are more susceptible to developing liver failure and having poor outcomes during anti-TB treatment. Regular monitoring of liver function and HBV DNA level is mandatory. Anti-HBV treatment should be considered in those with high viral levels before anti-TB treatment.
Keywords: Clinical outcome; Drug-induced liver injury; Hepatitis B virus; Liver failure; Tuberculosis.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the Third Affiliated Hospital of the Sun Yat-sen University, which waived the need for informed consent because all the data used in this retrospective study were routinely obtained and no additional procedures were carried out.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- World Health Organization. Global tuberculosis report 2017. Available from URL: http://who.int/tb/publications/global_report/en/. Accessed 28 June 2018.
-
- Wang LX, Chen SM, Chen MT, et al. The fifth national tuberculosis epidemiological survey in 2010 [Chinese] Chin J Antibiot. 2012;34(8):485–508.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

