Impact of universal home visits on maternal and infant outcomes in Bauchi state, Nigeria: protocol of a cluster randomized controlled trial
- PMID: 29970071
- PMCID: PMC6029180
- DOI: 10.1186/s12913-018-3319-z
Impact of universal home visits on maternal and infant outcomes in Bauchi state, Nigeria: protocol of a cluster randomized controlled trial
Abstract
Background: Maternal mortality in Nigeria is one of the highest in the world. Access to antenatal care is limited and the quality of services is poor in much of the country. Previous research in Bauchi State found associations between maternal morbidity and domestic violence, heavy work in pregnancy, lack of knowledge about danger signs, and lack of spousal communication about pregnancy and childbirth. This cluster randomized controlled stepped-wedge trial will test the impact of universal home visits to pregnant women and their partners, and the added value of video edutainment.
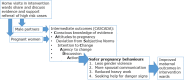
Methods: The trial will take place in six wards of Toro Local Government Area in Bauchi State, Nigeria, randomly allocated into three waves of two wards each. Home visits will begin in wave 1 wards immediately; in wave 2 wards after one year; and in wave 3 wards after a further year. In each wave, one ward, randomly allocated, will receive video edutainment during the home visits. Female home visitors will contact all households in their catchment areas of about 300 households, register all pregnant women, and visit them every two months during pregnancy, after delivery and one year later. They will use android handsets to collect information on pregnancy progress, send this to a central server, and discuss with the women the evidence about household factors associated with higher maternal risks, using video clips in the edutainment wards. Male home visitors will contact the partners of the pregnant women and discuss with them the same evidence. We will compare outcomes between wave 1 and wave 2 wards at about one year, between wave 2 and wave 3 wards at about two years, and finally between wards with and without added edutainment. Primary outcomes will be complications in pregnancy and delivery, and child health at one year. Secondary outcomes include knowledge and attitudes, use of health services, knowledge of danger signs, and household care of pregnant women.
Discussion: Demonstrating an impact of home visits and understanding potential mechanisms could have important implications for reducing maternal morbidity and mortality in other settings with poor access to quality antenatal care services.
Trial registration: Registration number: ISRCTN82954580 . Registry: ISRCTN. Date of registration: 11 August 2017. Retrospectively registered.
Keywords: Edutainment; Home visits; Male involvement; Maternal and newborn health; Nigeria; Randomized controlled trial; Stepped-wedge design.
Conflict of interest statement
Ethics approval and consent to participate
The trial has approval from the Bauchi State Health Research Ethics Committee (NREC/12/05/2015/12), on 12 May 2015, and from the McGill Faculty of Medicine IRB (A06-B35-15A), on 23 June 2015.
The research team in Bauchi will discuss the home visits with the leadership of all communities in the participating wards and get their approval to proceed. Fieldworkers will obtain oral informed consent from all female and male respondents in households for every interview. Using a script, they will explain to respondents the purpose of the visits, the confidentiality of responses, and the respondents’ rights not to answer certain questions or to terminate the interview. They will record the respondents’ informed consent on the android tablet at the beginning of every interview. For respondents under 16 years old, they will also obtain informed oral consent from the parent or guardian.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization. Trends in maternal mortality: 1990 to 2015. In: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: WHO; 2015. http://apps.who.int/iris/bitstream/handle/10665/194254/9789241565141_eng.... Accessed 29 June 2018.
-
- UNFPA. Unpublished program document based on routine data, 2006.
-
- World Health Organization. WHO recommendations on health promotion interventions for maternal and newborn health 2015. Geneva: WHO; 2015. http://apps.who.int/iris/bitstream/handle/10665/172427/9789241508742_rep.... Accessed 29 June 2018. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical