6-minute walk test as a measure of disease progression and fatigability in a cohort of individuals with RYR1-related myopathies
- PMID: 29970108
- PMCID: PMC6029052
- DOI: 10.1186/s13023-018-0848-9
6-minute walk test as a measure of disease progression and fatigability in a cohort of individuals with RYR1-related myopathies
Abstract
Background: RYR1-related Myopathies (RYR1-RM) comprise a group of rare neuromuscular diseases (NMDs) occurring in approximately 1/90000 people in the US pediatric population. RYR1-RM result from pathogenic mutations in the ryanodine receptor isoform-1 (RYR1) gene where consequent RyR1 protein calcium dysregulation leads to impaired excitation-contraction coupling, oxidative and nitrosative stress, and mitochondrial depletion. These physiological deficits perpetuate RyR1 dysfunction causing further muscle injury, muscle weakness, and muscle fatigue. Muscle weakness and fatigue are two primary complaints in patients with RYR1-RM and are major symptoms that limit the ability of individuals to perform activities of daily living. The six-minute walk test (6MWT) is an endurance test with high reliability and validity used to measure walking capacity, disease progression, and more recently, fatigability in NMDs with limited results in RYR1-RM. Therefore, the purpose of our study is to objectively assess disease progression and fatigability in RYR1-RM affected individuals using the 6MWT. We hypothesized that 6MWT distance and fatigability would not change significantly between 0 and 6-month visits in RYR1-RM patients, given the clinically reported stable or slowly progressive nature of the disease. We also hypothesized participants would show fatigability during the 6MWT, given muscle weakness and fatigue are the two primary complaints of affected individuals.
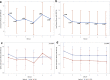
Results: As expected, paired t-test analyses revealed no significant difference between total distance traveled (p = .608) or percent change in speed (p = .141) at 0-months compared with the 6-month visit. Fatigability was observed given the decline in speed between the first and last minute of the 6MWT at the 6-month time point (p ≤ .0005,). Although this decline was not significant at baseline, a significant decline in speed from the 1st minute did occur at minutes 2, 3, and 4 during the baseline visit.
Conclusion: In this RYR1-RM cohort, the 6MWT showed disease stability over a 6-month period but revealed fatigability during the test. Given these results, the 6MWT may be a promising endpoint for evaluating fatigability and therapeutic efficacy in the 6-month treatment phase of our current n-acetylcysteine trial in this population. Improvement post intervention could be attributed to the intervention rather than variability in disease progression.
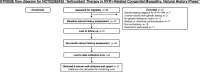
Trial registration: Clinical Trials.gov, NCT02362425 , Registered 13 February 2015-Prospectively registered.
Keywords: 6-min walk test; Disease progression; Fatigue; RyR1.
Conflict of interest statement
Ethics approval and consent to participate
This study (NCT02362425) and consent/assent forms were approved by the Institutional Review Board of the National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
There are no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

