Induction of immune response in chickens primed in ovo with an inactivated H9N2 avian influenza virus vaccine
- PMID: 29970157
- PMCID: PMC6029274
- DOI: 10.1186/s13104-018-3537-9
Induction of immune response in chickens primed in ovo with an inactivated H9N2 avian influenza virus vaccine
Abstract
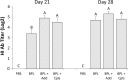
Objective: Infection of chickens with low pathogenic avian influenza virus, such as H9N2 virus, culminates in decreased egg production and increased mortality and morbidity if co-infection with other respiratory pathogens occurs. We have previously observed the induction of antibody- and cell-mediated immune responses after intramuscular administration of an H9N2 beta-propiolactone inactivated virus vaccine to chickens. Given the fact that in ovo vaccination represents a practical option for vaccination against H9N2 AIV in chickens, in the current study, we set out to characterize immune responses in chickens against a beta-propiolactone inactivated H9N2 virus vaccine after primary vaccination in ovo on embryonic day 18, and secondary intramuscular vaccination on day 14 post-hatch. We also included the Toll-like receptor 21 ligand, CpG ODN 2007, and an oil emulsion adjuvant, AddaVax™, as adjuvants for the vaccines.
Results: Antibody-mediated immune responses were observed after administering the secondary intramuscular vaccine. Cell-mediated immune responses were observed in chickens that received the beta-propiolactone inactivated H9N2 virus combined with AddaVax™. Our results demonstrate that adaptive immune responses can be induced in chickens after a primary in ovo vaccination and secondary intramuscular vaccination.
Keywords: Antibody; Beta-propiolactone; Cell-mediated; CpG ODN; H9N2 avian influenza virus; In ovo; Intramuscular; Toll-like receptor 21; Vaccine.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

