Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway
- PMID: 29970603
- PMCID: PMC6331045
- DOI: 10.1126/scisignal.aar5401
Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway
Abstract
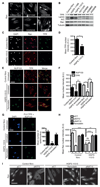
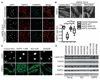
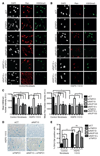
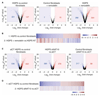
Hutchinson-Gilford progeria syndrome (HGPS) is an incurable premature aging disease. Identifying deregulated biological processes in HGPS might thus help define novel therapeutic strategies. Fibroblasts from HGPS patients display defects in nucleocytoplasmic shuttling of the GTP-bound form of the small GTPase Ran (RanGTP), which leads to abnormal transport of proteins into the nucleus. We report that microtubule stabilization in HGPS cells sequestered the nonclassical nuclear import protein Transportin-1 (TNPO1) in the cytoplasm, thus affecting the nuclear localization of its cargo, including the nuclear pore protein NUP153. Consequently, nuclear Ran, nuclear anchorage of the nucleoporin TPR, and chromatin organization were disrupted, deregulating gene expression and inducing senescence. Inhibiting N-acetyltransferase 10 (NAT10) ameliorated HGPS phenotypes by rebalancing the nuclear to cytoplasmic ratio of TNPO1. This restored nuclear pore complex integrity and nuclear Ran localization, thereby correcting HGPS cellular phenotypes. We observed a similar mechanism in cells from healthy aged individuals. This study identifies a nuclear import pathway affected in aging and underscores the potential for NAT10 inhibition as a possible therapeutic strategy for HGPS and perhaps also for pathologies associated with normal aging.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Nuclear import pathway key to rescuing dominant progerin phenotypes.Sci Signal. 2018 Jul 3;11(537):eaat9448. doi: 10.1126/scisignal.aat9448. Sci Signal. 2018. PMID: 29970604 Free PMC article.
References
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Lévy N. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. - PubMed
-
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: Building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. - PubMed
-
- Liu B, Wang J, Chan K Ming, Tjia W Mui, Deng W, Guan X, Huang J, Li K Man, Chau P Yin, Chen DJ, Pei D, et al. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 206242/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- 86609/WT_/Wellcome Trust/United Kingdom
- 092096/WT_/Wellcome Trust/United Kingdom
- 18796/CRUK_/Cancer Research UK/United Kingdom
- 10827/CRUK_/Cancer Research UK/United Kingdom
- C6946/A24843/CRUK_/Cancer Research UK/United Kingdom
- C6/A18796/CRUK_/Cancer Research UK/United Kingdom
- 17001/CRUK_/Cancer Research UK/United Kingdom
- 206388/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- WT203144/WT_/Wellcome Trust/United Kingdom
- RG17001/MRC_/Medical Research Council/United Kingdom
- MR/L019116/1/MRC_/Medical Research Council/United Kingdom
- 11224/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

