The Presence of Toxic and Non-Toxic Cyanobacteria in the Sediments of the Limpopo River Basin: Implications for Human Health
- PMID: 29970791
- PMCID: PMC6071004
- DOI: 10.3390/toxins10070269
The Presence of Toxic and Non-Toxic Cyanobacteria in the Sediments of the Limpopo River Basin: Implications for Human Health
Abstract
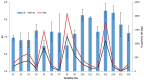
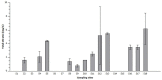
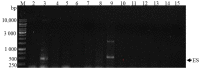
The presence of harmful algal blooms (HABs) and cyanotoxins in drinking water sources poses a great threat to human health. The current study employed molecular techniques to determine the occurrence of non-toxic and toxic cyanobacteria species in the Limpopo River basin based on the phylogenetic analysis of the 16S rRNA gene. Bottom sediment samples were collected from selected rivers: Limpopo, Crocodile, Mokolo, Mogalakwena, Nzhelele, Lephalale, Sand Rivers (South Africa); Notwane (Botswana); and Shashe River and Mzingwane River (Zimbabwe). A physical-chemical analysis of the bottom sediments showed the availability of nutrients, nitrates and phosphates, in excess of 0.5 mg/L, in most of the river sediments, while alkalinity, pH and salinity were in excess of 500 mg/L. The FlowCam showed the dominant cyanobacteria species that were identified from the sediment samples, and these were the Microcystis species, followed by Raphidiopsis raciborskii, Phormidium and Planktothrix species. The latter species were also confirmed by molecular techniques. Nevertheless, two samples showed an amplification of the cylindrospermopsin polyketide synthetase gene (S3 and S9), while the other two samples showed an amplification for the microcystin/nodularin synthetase genes (S8 and S13). Thus, these findings may imply the presence of toxic cyanobacteria species in the studied river sediments. The presence of cyanobacteria may be hazardous to humans because rural communities and farmers abstract water from the Limpopo river catchment for human consumption, livestock and wildlife watering and irrigation.
Keywords: PCR; akinetes; cyanobacteria; cyanotoxins; harmful algal blooms; nutrient enrichment; phylogenetic analyses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Microbiological and 16S rRNA analysis of sulphite-reducing clostridia from river sediments in central Italy.BMC Microbiol. 2008 Oct 8;8:171. doi: 10.1186/1471-2180-8-171. BMC Microbiol. 2008. PMID: 18842132 Free PMC article.
-
Occurrence and toxicity of Microcystis aeruginosa (Cyanobacteria) in the Paraná River, downstream of the Yacyretá dam (Argentina).Rev Biol Trop. 2016 Mar;64(1):203-11. Rev Biol Trop. 2016. PMID: 28862419
-
Temporal shifts in cyanobacterial communities at different sites on the Nakdong River in Korea.Water Res. 2013 Dec 1;47(19):6973-82. doi: 10.1016/j.watres.2013.09.058. Epub 2013 Oct 20. Water Res. 2013. PMID: 24169512
-
Application of ancient DNA to the reconstruction of past microbial assemblages and for the detection of toxic cyanobacteria in subtropical freshwater ecosystems.Mol Ecol. 2014 Dec;23(23):5791-802. doi: 10.1111/mec.12979. Epub 2014 Nov 21. Mol Ecol. 2014. PMID: 25346253
-
Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency event.PLoS One. 2018 May 23;13(5):e0196278. doi: 10.1371/journal.pone.0196278. eCollection 2018. PLoS One. 2018. PMID: 29791446 Free PMC article.
Cited by
-
Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection.Toxins (Basel). 2020 Jun 2;12(6):368. doi: 10.3390/toxins12060368. Toxins (Basel). 2020. PMID: 32498435 Free PMC article. Review.
-
The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review.Toxins (Basel). 2019 Sep 12;11(9):530. doi: 10.3390/toxins11090530. Toxins (Basel). 2019. PMID: 31547379 Free PMC article.
-
A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs.Toxins (Basel). 2019 Dec 20;12(1):6. doi: 10.3390/toxins12010006. Toxins (Basel). 2019. PMID: 31861931 Free PMC article. Review.
-
Understanding the Risks of Diffusion of Cyanobacteria Toxins in Rivers, Lakes, and Potable Water.Toxins (Basel). 2023 Sep 20;15(9):582. doi: 10.3390/toxins15090582. Toxins (Basel). 2023. PMID: 37756009 Free PMC article. Review.
-
Cyanobacterial Akinete Distribution, Viability, and Cyanotoxin Records in Sediment Archives From the Northern Baltic Sea.Front Microbiol. 2021 Jun 15;12:681881. doi: 10.3389/fmicb.2021.681881. eCollection 2021. Front Microbiol. 2021. PMID: 34211448 Free PMC article.
References
-
- Janse I., Kardinaal W.E.A., Meima M., Fastner J., Visser P.M., Zwart G. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl. Environ. Microbiol. 2004;70:3979–3987. doi: 10.1128/AEM.70.7.3979-3987.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

