Transcription Factor ETS-1 and Reactive Oxygen Species: Role in Vascular and Renal Injury
- PMID: 29970819
- PMCID: PMC6071050
- DOI: 10.3390/antiox7070084
Transcription Factor ETS-1 and Reactive Oxygen Species: Role in Vascular and Renal Injury
Abstract
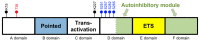
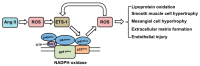
The E26 avian erythroblastosis virus transcription factor-1 (ETS-1) is a member of the ETS family and regulates the expression of a variety of genes including growth factors, chemokines and adhesion molecules. Although ETS-1 was discovered as an oncogene, several lines of research show that it is up-regulated by angiotensin II (Ang II) both in the vasculature and the glomerulus. While reactive oxygen species (ROS) are required for Ang II-induced ETS-1 expression, ETS-1 also regulates the expression of p47phox, which is one of the subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and a major source of ROS in the kidney and vasculature. Thus, there appears to be a positive feedback between ETS-1 and ROS. ETS-1 is also upregulated in the kidneys of rats with salt-sensitive hypertension and plays a major role in the development of end-organ injury in this animal model. Activation of the renin angiotensin system is required for the increased ETS-1 expression in these rats, and blockade of ETS-1 or haplodeficiency reduces the severity of kidney injury in these rats. In summary, ETS-1 plays a major role in the development of vascular and renal injury and is a potential target for the development of novel therapeutic strategies to ameliorate end-organ injury in hypertension.
Keywords: ETS-1; reactive oxygen species; renal injury; vascular injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Transcription factor avian erythroblastosis virus E26 oncogen homolog-1 is a novel mediator of renal injury in salt-sensitive hypertension.Hypertension. 2015 Apr;65(4):813-20. doi: 10.1161/HYPERTENSIONAHA.114.04533. Epub 2015 Jan 26. Hypertension. 2015. PMID: 25624342 Free PMC article.
-
Role of the transcription factor erythroblastosis virus E26 oncogen homolog-1 (ETS-1) as mediator of the renal proinflammatory and profibrotic effects of angiotensin II.Hypertension. 2012 Nov;60(5):1226-33. doi: 10.1161/HYPERTENSIONAHA.112.197871. Epub 2012 Sep 10. Hypertension. 2012. PMID: 22966006 Free PMC article.
-
Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells.Am J Physiol Renal Physiol. 2008 May;294(5):F1094-100. doi: 10.1152/ajprenal.00458.2007. Epub 2008 Mar 12. Am J Physiol Renal Physiol. 2008. PMID: 18337545
-
[Pathophysiological and clinical implications of AT(1) and AT(2) angiotensin II receptors in metabolic disorders: hypercholesterolaemia and diabetes].Drugs. 2002;62 Spec No 1:31-41. Drugs. 2002. PMID: 12036387 Review. French.
-
Suppressing renal NADPH oxidase to treat diabetic nephropathy.Expert Opin Ther Targets. 2007 Aug;11(8):1011-8. doi: 10.1517/14728222.11.8.1011. Expert Opin Ther Targets. 2007. PMID: 17665974 Review.
Cited by
-
HUWE1 Causes an Immune Imbalance in Immune Thrombocytopenic Purpura by Reducing the Number and Function of Treg Cells Through the Ubiquitination Degradation of Ets-1.Front Cell Dev Biol. 2021 Nov 25;9:708562. doi: 10.3389/fcell.2021.708562. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34900980 Free PMC article.
-
Discovery of ETS1 as a New Gene Predisposing to Dilated Cardiomyopathy.Diagnostics (Basel). 2025 Aug 13;15(16):2031. doi: 10.3390/diagnostics15162031. Diagnostics (Basel). 2025. PMID: 40870883 Free PMC article.
-
ETS-1 in tumor immunology: implications for novel anti-cancer strategies.Front Immunol. 2025 Mar 20;16:1526368. doi: 10.3389/fimmu.2025.1526368. eCollection 2025. Front Immunol. 2025. PMID: 40181983 Free PMC article. Review.
-
High salt-induced weakness of anti-oxidative function of natriuretic peptide receptor-C and podocyte damage in the kidneys of Dahl rats.Chin Med J (Engl). 2020 May 20;133(10):1182-1191. doi: 10.1097/CM9.0000000000000752. Chin Med J (Engl). 2020. PMID: 32433050 Free PMC article.
-
Signal Transduction and Gene Regulation in the Endothelium.Cold Spring Harb Perspect Med. 2023 Jan 3;13(1):a041153. doi: 10.1101/cshperspect.a041153. Cold Spring Harb Perspect Med. 2023. PMID: 35667710 Free PMC article. Review.
References
-
- Watson D.K., McWilliams-Smith M.J., Nunn M.F., Duesberg P.H., O’Brien S.J., Papas T.S. The ets sequence from the transforming gene of avian erythroblastosis virus, E26, has unique domains on human chromosomes 11 and 21: Both loci are transcriptionally active. Proc. Natl. Acad. Sci. USA. 1985;82:7294–7298. doi: 10.1073/pnas.82.21.7294. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

