Tracking Cell Recruitment and Behavior within the Tumor Microenvironment Using Advanced Intravital Imaging Approaches
- PMID: 29970845
- PMCID: PMC6071013
- DOI: 10.3390/cells7070069
Tracking Cell Recruitment and Behavior within the Tumor Microenvironment Using Advanced Intravital Imaging Approaches
Abstract
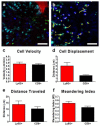
Recent advances in imaging technology have made it possible to track cellular recruitment and behavior within the vasculature of living animals in real-time. Using approaches such as resonant scanning confocal and multiphoton intravital microscopy (IVM), we are now able to observe cells within the intact tumor microenvironment of a mouse. We are able to follow these cells for extended periods of time (hours) and can characterize how specific cell types (T cells, neutrophils, monocytes) interact with the tumor vasculature and cancer cells. This approach provides greater insight into specific cellular behaviors and cell⁻cell interactions than conventional techniques such as histology and flow cytometry. In this report, we describe the surgical preparation of animals to expose the tumor and both resonant scanning confocal and multiphoton imaging approaches used to track leukocyte recruitment, adhesion, and behavior within the tumor microenvironment. We present techniques for the measurement and quantification of leukocyte behavior within the bloodstream and tumor interstitium. The use of IVM to study leukocyte behavior within the tumor microenvironment provides key information not attainable with other approaches, that will help shape the development of better, more effective anticancer drugs and therapeutic approaches.
Keywords: cancer; imaging; intravital; leukocytes; trafficking; vasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cohnheim J. Untersuchungen über die Embolischen Process. August Hirschwald; Berlin, Germany: 1872.
-
- Waller A. Microscopic observations on the perforation of the capillaries by the corpuscles of the blood, and on the origin of mucus and pus-globules. Lond. Edinb. Dublin Philos. Mag. 1846;29:397–405. doi: 10.1080/14786444608645527. - DOI
-
- Hall M. A Critical and Experimental Essay on the Circulation of the Blood. R.B. Seeley and W. Burnside; London, UK: 1831.
LinkOut - more resources
Full Text Sources
Other Literature Sources

