Pyruvate kinase M2 isoform deletion in cone photoreceptors results in age-related cone degeneration
- PMID: 29970877
- PMCID: PMC6030055
- DOI: 10.1038/s41419-018-0712-9
Pyruvate kinase M2 isoform deletion in cone photoreceptors results in age-related cone degeneration
Abstract
The tumor form of pyruvate kinase M2 has been suggested to promote cellular anabolism by redirecting the metabolism to cause accumulation of glycolytic intermediates and increasing flux through the pentose phosphate pathway, which is a metabolic pathway parallel to glycolysis. Both rod and cone photoreceptors express the tumor form of pyruvate kinase M2. Recent studies from our laboratory show that PKM2 is functionally important for rod photoreceptor structure, function, and viability. However, the functional role of PKM2 in cones is not known. In this study, we conditionally deleted PKM2 in cones (cone-cre PKM2-KO) and found that loss of PKM2 results in the upregulation of PKM1 and a significant loss of cone function and cone degeneration in an age-dependent manner. Gene expression studies on cone-cre PKM2-KO show decreased expression of genes regulating glycolysis, PPP shunt, and fatty acid biosynthesis. Consistent with these observations, cones lacking PKM2 have significantly shorter cone outer segments than cones with PKM2. Our studies clearly suggest that PKM2 is essential for the anabolic process in cones to keep them alive for normal functioning and to support cone structure.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


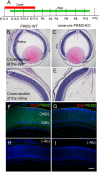
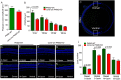


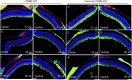
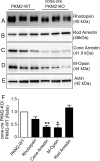


References
-
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol. Vis. Sci. 1985;26:1659–1694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

