Local and Widespread Slow Waves in Stable NREM Sleep: Evidence for Distinct Regulation Mechanisms
- PMID: 29970995
- PMCID: PMC6018150
- DOI: 10.3389/fnhum.2018.00248
Local and Widespread Slow Waves in Stable NREM Sleep: Evidence for Distinct Regulation Mechanisms
Abstract
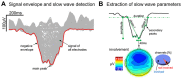
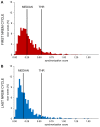
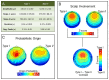
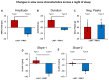
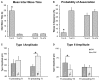
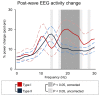
Previous work showed that two types of slow waves are temporally dissociated during the transition to sleep: widespread, large and steep slow waves predominate early in the falling asleep period (type I), while smaller, more circumscribed slow waves become more prevalent later (type II). Here, we studied the possible occurrence of these two types of slow waves in stable non-REM (NREM) sleep and explored potential differences in their regulation. A heuristic approach based on slow wave synchronization efficiency was developed and applied to high-density electroencephalographic (EEG) recordings collected during consolidated NREM sleep to identify the potential type I and type II slow waves. Slow waves with characteristics compatible with those previously described for type I and type II were identified in stable NREM sleep. Importantly, these slow waves underwent opposite changes across the night, with only type II slow waves displaying a clear homeostatic regulation. In addition, we showed that the occurrence of type I slow waves was often followed by larger type II slow waves, whereas the occurrence of type II slow waves was usually followed by smaller type I waves. Finally, type II slow waves were associated with a relative increase in spindle activity, while type I slow waves triggered periods of high-frequency activity. Our results provide evidence for the existence of two distinct slow wave synchronization processes that underlie two different types of slow waves. These slow waves may have different functional roles and mark partially distinct "micro-states" of the sleeping brain.
Keywords: K-complex; NREM sleep; high-density EEG; slow wave activity; slow waves.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

