A Controlled Fermented Samjunghwan Herbal Formula Ameliorates Non-alcoholic Hepatosteatosis in HepG2 Cells and OLETF Rats
- PMID: 29971000
- PMCID: PMC6018163
- DOI: 10.3389/fphar.2018.00596
A Controlled Fermented Samjunghwan Herbal Formula Ameliorates Non-alcoholic Hepatosteatosis in HepG2 Cells and OLETF Rats
Abstract
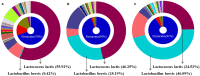
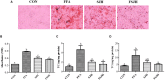
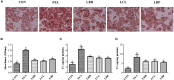
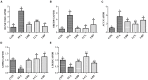
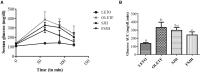
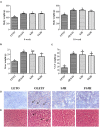
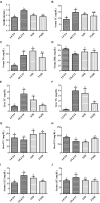
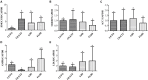
Hepatosteatosis (HS), a clinical feature of fatty liver with the excessive intracellular accumulation of triglyceride in hepatocytes, is manifested by perturbation of the maintenance of liver lipid homeostasis. Samjunghwan (SJH) is an herbal formula used mostly in Korean traditional medicine that is effective against a number of metabolic diseases, including obesity. Herbal drugs, enriched with numerous bioactive substances, possess health-protective benefits. Meanwhile, fermented herbal products enriched with probiotics are known to improve metabolic processes. Additionally, current lines of evidence indicate that probiotics-derived metabolites, termed as postbiotics, produce the same beneficial effects as their precursors. Herein, the anti-HS effects of 5-weeks naturally fermented SJH (FSJH) was investigated with FSJH-mixed chow diet in vivo using Otsuka Long-Evans Tokushima Fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) rats as animal models of HS and controls, respectively. In parallel, the anti-HS effects of postbiotic-metabolites of three bacterial strains [Lactobacillus brevis (LBB), Lactococcus lactis (LCL) and Lactobacillus plantarum (LBP)] isolated from FSJH were also evaluated in vitro using the FFAs-induced HepG2 cells. Feeding OLETF rats with FSJH-diet effectively reduced body, liver, and visceral adipose tissue (VAT) weights, produced marked hypolipidemic effects on serum and hepatic lipid parameters, decreased serum AST and ALT levels, and upregulated the HMGCOR, SREBP, and ACC, and downregulated the AMPK and LDLR gene expressions levels. Additionally, exposure of FFAs-induced HepG2 cells to postbiotic metabolic media (PMM) of bacterial strains also produced marked hypolipidemic effects on intracellular lipid contents and significantly unregulated the HMGCOR, SREBP, and ACC, and downregulated the AMPK and LDLR genes expressions levels. Overall, our results indicate that FSJH enriched with fermented metabolites could be an effective anti-HS formulation.
Keywords: Lactobacillus bravis; Lactobacillus lactis; Lactobacillus plantarum; Samjunghwan; fermentation; hepatosteatosis; postbiotic.
Figures


References
-
- Bradbury M. W. (2006). Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 290 G194–G198. - PubMed
-
- Chang S., Wang J.-H., Shin N. R., Kim H. (2016). Microbiological characteristics and cytoprotective effects of samjung-hwan fermented by lactic acid bacteria. J. Korean Med. Obes. Res. 16 11–18. 10.15429/jkomor.2016.16.1.11 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

