Validation of Patient-Specific Cerebral Blood Flow Simulation Using Transcranial Doppler Measurements
- PMID: 29971012
- PMCID: PMC6018476
- DOI: 10.3389/fphys.2018.00721
Validation of Patient-Specific Cerebral Blood Flow Simulation Using Transcranial Doppler Measurements
Abstract
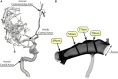
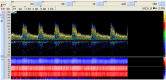
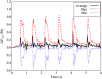
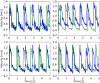
We present a validation study comparing results from a patient-specific lattice-Boltzmann simulation to transcranial Doppler (TCD) velocity measurements in four different planes of the middle cerebral artery (MCA). As part of the study, we compared simulations using a Newtonian and a Carreau-Yasuda rheology model. We also investigated the viability of using downscaled velocities to reduce the required resolution. Simulations with unscaled velocities predict the maximum flow velocity with an error of less than 9%, independent of the rheology model chosen. The accuracy of the simulation predictions worsens considerably when simulations are run at reduced velocity, as is for example the case when inflow velocities from healthy individuals are used on a vascular model of a stroke patient. Our results demonstrate the importance of using directly measured and patient-specific inflow velocities when simulating blood flow in MCAs. We conclude that localized TCD measurements together with predictive simulations can be used to obtain flow estimates with high fidelity over a larger region, and reduce the need for more invasive flow measurement procedures.
Keywords: blood flow; computational fluid dynamics; high performance computing; lattice-Boltzmann; middle cerebral artery; transcranial Doppler; validation study.
Figures



References
-
- Bernsdorf J., Wang D. (2009). Non-Newtonian blood flow simulation in cerebral aneurysms. Comput. Math. Appl. 58, 1024–1029. 10.1016/j.camwa.2009.02.019 - DOI
-
- Bhatnagar P. L., Gross E. P., Krook M. (1954). A model for collision processes in gases. I. Small amplitude processes in charged and neutral one-component systems. Phys. Rev. 94, 511–525.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

