From Host Heme To Iron: The Expanding Spectrum of Heme Degrading Enzymes Used by Pathogenic Bacteria
- PMID: 29971218
- PMCID: PMC6018153
- DOI: 10.3389/fcimb.2018.00198
From Host Heme To Iron: The Expanding Spectrum of Heme Degrading Enzymes Used by Pathogenic Bacteria
Abstract
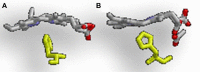
Iron is an essential nutrient for many bacteria. Since the metal is highly sequestered in host tissues, bound predominantly to heme, pathogenic bacteria often take advantage of heme uptake and degradation mechanisms to acquire iron during infection. The most common mechanism of releasing iron from heme is through oxidative degradation by heme oxygenases (HOs). In addition, an increasing number of proteins that belong to two distinct structural families have been implicated in aerobic heme catabolism. Finally, an enzyme that degrades heme anaerobically was recently uncovered, further expanding the mechanisms for bacterial heme degradation. In this analysis, we cover the spectrum and recent advances in heme degradation by infectious bacteria. We briefly explain heme oxidation by the two groups of recognized HOs to ground readers before focusing on two new types of proteins that are reported to be involved in utilization of heme iron. We discuss the structure and enzymatic function of proteins representing these groups, their biological context, and how they are regulated to provide a more complete look at their cellular role.
Keywords: heme binding; heme degradation; heme oxygenase; iron regulation; pathogenic bacteria.
Figures



Similar articles
-
Heme utilization by pathogenic bacteria: not all pathways lead to biliverdin.Acc Chem Res. 2014 Aug 19;47(8):2291-8. doi: 10.1021/ar500028n. Epub 2014 May 29. Acc Chem Res. 2014. PMID: 24873177 Free PMC article.
-
Bacterial heme oxygenases.Antioxid Redox Signal. 2004 Oct;6(5):825-34. doi: 10.1089/ars.2004.6.825. Antioxid Redox Signal. 2004. PMID: 15345142 Review.
-
Structural and biochemical study of Bacillus subtilis HmoB in complex with heme.Biochem Biophys Res Commun. 2014 Mar 28;446(1):286-91. doi: 10.1016/j.bbrc.2014.02.092. Epub 2014 Feb 28. Biochem Biophys Res Commun. 2014. PMID: 24582752
-
Bacillus subtilis HmoB is a heme oxygenase with a novel structure.BMB Rep. 2012 Apr;45(4):239-41. doi: 10.5483/bmbrep.2012.45.4.239. BMB Rep. 2012. PMID: 22531134
-
Heme oxygenase: evolution, structure, and mechanism.Antioxid Redox Signal. 2002 Aug;4(4):603-14. doi: 10.1089/15230860260220102. Antioxid Redox Signal. 2002. PMID: 12230872 Review.
Cited by
-
Heme utilization by the enterococci.FEMS Microbes. 2024 Jul 2;5:xtae019. doi: 10.1093/femsmc/xtae019. eCollection 2024. FEMS Microbes. 2024. PMID: 39070772 Free PMC article. Review.
-
The impact of iron and heme availability on the healthy human gut microbiome in vivo and in vitro.Cell Chem Biol. 2023 Jan 19;30(1):110-126.e3. doi: 10.1016/j.chembiol.2022.12.001. Epub 2023 Jan 4. Cell Chem Biol. 2023. PMID: 36603582 Free PMC article.
-
Heme Binding to HupZ with a C-Terminal Tag from Group A Streptococcus.Molecules. 2021 Jan 21;26(3):549. doi: 10.3390/molecules26030549. Molecules. 2021. PMID: 33494451 Free PMC article.
-
Heme alters biofilm formation in Mycobacterium abscessus.Microbiol Spectr. 2025 Feb 4;13(2):e0241524. doi: 10.1128/spectrum.02415-24. Epub 2024 Dec 23. Microbiol Spectr. 2025. PMID: 39705014 Free PMC article.
-
Biosynthesis of the modified tetrapyrroles-the pigments of life.J Biol Chem. 2020 May 15;295(20):6888-6925. doi: 10.1074/jbc.REV120.006194. Epub 2020 Apr 2. J Biol Chem. 2020. PMID: 32241908 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

