Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial
- PMID: 29971397
- PMCID: PMC6583046
- DOI: 10.1001/jama.2018.7464
Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial
Erratum in
-
Clarification for Reported Colposcopy Rates.JAMA. 2018 Dec 4;320(21):2273. doi: 10.1001/jama.2018.17912. JAMA. 2018. PMID: 30512084 Free PMC article. No abstract available.
Abstract
Importance: There is limited information about the relative effectiveness of cervical cancer screening with primary human papillomavirus (HPV) testing alone compared with cytology in North American populations.
Objective: To evaluate histologically confirmed cumulative incident cervical intraepithelial neoplasia (CIN) grade 3 or worse (CIN3+) detected up to and including 48 months by primary HPV testing alone (intervention) or liquid-based cytology (control).
Design, setting, and participants: Randomized clinical trial conducted in an organized Cervical Cancer Screening Program in Canada. Participants were recruited through 224 collaborating clinicians from January 2008 to May 2012, with follow-up through December 2016. Women aged 25 to 65 years with no history of CIN2+ in the past 5 years, no history of invasive cervical cancer, or no history of hysterectomy; who have not received a Papanicolaou test within the past 12 months; and who were not receiving immunosuppressive therapy were eligible.
Interventions: A total of 19 009 women were randomized to the intervention (n = 9552) and control (n = 9457) groups. Women in the intervention group received HPV testing; those whose results were negative returned at 48 months. Women in the control group received liquid-based cytology (LBC) testing; those whose results were negative returned at 24 months for LBC. Women in the control group who were negative at 24 months returned at 48 months. At 48-month exit, both groups received HPV and LBC co-testing.
Main outcomes and measures: The primary outcome was the cumulative incidence of CIN3+ 48 months following randomization. The cumulative incidence of CIN2+ was a secondary outcome.
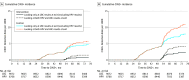
Results: Among 19 009 women who were randomized (mean age, 45 years [10th-90th percentile, 30-59]), 16 374 (8296 [86.9%] in the intervention group and 8078 [85.4%] in the control group) completed the study. At 48 months, significantly fewer CIN3+ and CIN2+ were detected in the intervention vs control group. The CIN3+ incidence rate was 2.3/1000 (95% CI, 1.5-3.5) in the intervention group and 5.5/1000 (95% CI, 4.2-7.2) in the control group. The CIN3+ risk ratio was 0.42 (95% CI, 0.25-0.69). The CIN2+ incidence rate at 48 months was 5.0/1000 (95% CI, 3.8-6.7) in the intervention group and 10.6/1000 (95% CI, 8.7-12.9) in the control group. The CIN2+ risk ratio was 0.47 (95% CI, 0.34-0.67). Baseline HPV-negative women had a significantly lower cumulative incidence of CIN3+ at 48 months than cytology-negative women (CIN3+ incidence rate, 1.4/1000 [95% CI, 0.8-2.4]; CIN3+ risk ratio, 0.25 [95% CI, 0.13-0.48]).
Conclusions and relevance: Among women undergoing cervical cancer screening, the use of primary HPV testing compared with cytology testing resulted in a significantly lower likelihood of CIN3+ at 48 months. Further research is needed to understand long-term clinical outcomes as well as cost-effectiveness.
Trial registration: isrctn.org Identifier: ISRCTN79347302.
Conflict of interest statement
Figures


Comment in
-
Replacing the Pap Test With Screening Based on Human Papillomavirus Assays.JAMA. 2018 Jul 3;320(1):35-37. doi: 10.1001/jama.2018.7911. JAMA. 2018. PMID: 29971379 No abstract available.
-
HPV Testing Bests Pap for Cervical Screening.Cancer Discov. 2018 Sep;8(9):OF6. doi: 10.1158/2159-8290.CD-NB2018-100. Epub 2018 Jul 27. Cancer Discov. 2018. PMID: 30054288
-
Human papillomavirus testing may detect cervical precancer better than Papanicolaou testing.Cancer. 2018 Dec 1;124(23):4430-4431. doi: 10.1002/cncr.31867. Cancer. 2018. PMID: 30536585 No abstract available.
-
Initial Screening for Cervical Intraepithelial Neoplasia.Am J Clin Pathol. 2019 Jul 5;152(2):253. doi: 10.1093/ajcp/aqz058. Am J Clin Pathol. 2019. PMID: 31227830 No abstract available.
References
-
- American Society of Clinical Oncology . Cervical cancer: statistics. Cancer.net. http://www.cancer.net/cancer-types/cervical-cancer/statistics. Published July 2017. Accessed April 14, 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

