Biomechanical and morphological stability of acellular scaffolds for tissue-engineered heart valves depends on different storage conditions
- PMID: 29971508
- PMCID: PMC6028870
- DOI: 10.1007/s10856-018-6106-9
Biomechanical and morphological stability of acellular scaffolds for tissue-engineered heart valves depends on different storage conditions
Abstract
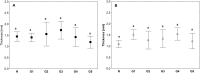
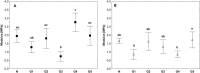
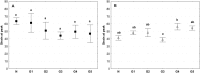
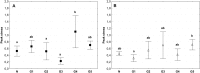
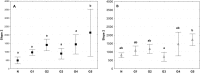
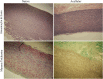
Currently available bioprosthetic heart valves have been successfully used clinically; however, they have several limitations. Alternatively, tissue-engineering techniques can be used. However, there are limited data concerning the impact of storage conditions of scaffolds on their biomechanics and morphology. The aim of this study was to determine the effect of different storage conditions on the biomechanics and morphology of pulmonary valve dedicated for the acellular scaffold preparation to achieve optimal conditions to obtain stable heart valve prostheses. Scaffold can then be used for the construction of tissue-engineered heart valve, for this reason evaluation of these parameters can determine the success of the clinical application this type of bioprosthesis. Pulmonary heart valves were collected from adult porcines. Materials were divided into five groups depending on the storage conditions. Biomechanical tests were performed, both the static tensile test, and examination of viscoelastic properties. Extracellular matrix morphology was evaluated using transmission electron microscopy and immunohistochemistry. Tissue stored at 4 °C exhibited a higher modulus of elasticity than the control (native) and fresh acellular, which indicated the stiffening of the tissue and changes of the viscoelastic properties. Such changes were not observed in the radial direction. Percent strain was not significantly different in the study groups. The storage conditions affected the acellularization efficiency and tissue morphology. To the best of our knowledge, this study is the first that attributes the mechanical properties of pulmonary valve tissue to the biomechanical changes in the collagen network due to different storage conditions. Storage conditions of scaffolds for tissue-engineered heart valves may have a significant impact on the haemodynamic and clinical effects of the used bioprostheses.
Conflict of interest statement
The authors certify that they have NO affiliations with or involvement in any organization or entity withany financial interest (such as honoraria; educational grants; participation in speakers’ bureaus;membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony orpatent-licensing arrangements), or non-financial interest (such as personal or professional relationships,affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures












References
-
- World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. ESA/P/WP/248. United Nations, Department of Economic and Social Affairs, Population Division. 2017.
-
- Tofield A. European cardiovascular disease statistics 4th edition 2012: EuroHeart II. Eur Heart J. 2013;34:3007–14. doi: 10.1093/eurheartj/eht379. - DOI
-
- Marijon E, Celermajer DS, Tafflet M, El-Haou S, Jani DN, Ferreira B, et al. Rheumatic heart disease screening by echocardiography the inadequacy of world health organization criteria for optimizing the diagnosis of subclinical disease. Circulation. 2009;120:663–8. doi: 10.1161/CIRCULATIONAHA.109.849190. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

