Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis
- PMID: 29971947
- PMCID: PMC6111865
- DOI: 10.1111/jcmm.13725
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis
Abstract
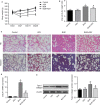
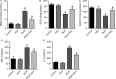
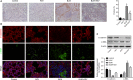
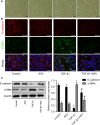
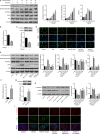
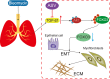
Epithelial-mesenchymal transition (EMT) plays an important role in idiopathic pulmonary fibrosis (IPF). Astragaloside IV (ASV), a natural saponin from astragalus membranaceus, has shown anti-fibrotic property in bleomycin (BLM)-induced pulmonary fibrosis. The current study was undertaken to determine whether EMT was involved in the beneficial of ASV against BLM-induced pulmonary fibrosis and to elucidate its potential mechanism. As expected, in BLM-induced IPF, ASV exerted protective effects on pulmonary fibrosis and ASV significantly reversed BLM-induced EMT. Intriguing, transforming growth factor-β1 (TGF-β1) was found to be up-regulated, whereas Forkhead box O3a (FOXO3a) was hyperphosphorylated and less expressed. However, ASV treatment inhibited increased TGF-β1 and activated FOXO3a in lung tissues. TGF-β1 was administered to alveolar epithelial cells A549 to induce EMT in vitro. Meanwhile, stimulation with TGF-β1-activated phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) pathway and induced FOXO3a hyperphosphorylated and down-regulated. It was found that overexpression of FOXO3a leading to the suppression of TGF-β1-induced EMT. Moreover, ASV treatment, similar with the TGF-β1 or PI3K/Akt inhibitor, reverted these cellular changes and inhibited EMT in A549 cells. Collectively, the results suggested that ASV significantly inhibited TGF-β1/PI3K/Akt-induced FOXO3a hyperphosphorylation and down-regulation to reverse EMT during the progression of fibrosis.
Keywords: astragaloside IV; epithelial-mesenchymal transition; forkhead box O3a; idiopathic pulmonary fibrosis; transforming growth factor-β1.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
ESL attenuates BLM-induced IPF in mice: Dual mediation of the TLR4/NF-κB and TGF-β1/PI3K/Akt/FOXO3a pathways.Phytomedicine. 2024 Sep;132:155545. doi: 10.1016/j.phymed.2024.155545. Epub 2024 Apr 2. Phytomedicine. 2024. PMID: 38972238
-
Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells.Phytother Res. 2018 Jul;32(7):1289-1296. doi: 10.1002/ptr.6057. Epub 2018 Feb 26. Phytother Res. 2018. PMID: 29480652
-
Metastasis-associated protein 1 promotes epithelial-mesenchymal transition in idiopathic pulmonary fibrosis by up-regulating Snail expression.J Cell Mol Med. 2020 Jun;24(11):5998-6007. doi: 10.1111/jcmm.15062. Epub 2020 Mar 18. J Cell Mol Med. 2020. PMID: 32187849 Free PMC article.
-
MicroRNAs in idiopathic pulmonary fibrosis.Transl Res. 2011 Apr;157(4):191-9. doi: 10.1016/j.trsl.2011.01.012. Epub 2011 Feb 4. Transl Res. 2011. PMID: 21420029 Review.
-
[Research progress of anti-fibrotic drugs that inhibit epithelial-mesenchymal transition in pulmonary fibrosis].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023 Jan 20;41(1):72-77. doi: 10.3760/cma.j.cn121094-20210628-00308. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023. PMID: 36725301 Review. Chinese.
Cited by
-
The mechanism and application of traditional Chinese medicine extracts in the treatment of lung cancer and other lung-related diseases.Front Pharmacol. 2023 Dec 6;14:1330518. doi: 10.3389/fphar.2023.1330518. eCollection 2023. Front Pharmacol. 2023. PMID: 38125887 Free PMC article. Review.
-
Astragaloside IV protects endothelial progenitor cells from the damage of ox-LDL via the LOX-1/NLRP3 inflammasome pathway.Drug Des Devel Ther. 2019 Jul 29;13:2579-2589. doi: 10.2147/DDDT.S207774. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31440038 Free PMC article.
-
Vitamin D deficiency exacerbates bleomycin-induced pulmonary fibrosis partially through aggravating TGF-β/Smad2/3-mediated epithelial-mesenchymal transition.Respir Res. 2019 Nov 27;20(1):266. doi: 10.1186/s12931-019-1232-6. Respir Res. 2019. PMID: 31775746 Free PMC article.
-
Astragaloside IV inhibits oxidized low‑density lipoprotein‑induced endothelial damage via upregulation of miR‑140‑3p.Int J Mol Med. 2019 Sep;44(3):847-856. doi: 10.3892/ijmm.2019.4257. Epub 2019 Jun 26. Int J Mol Med. 2019. Retraction in: Int J Mol Med. 2023 Feb;51(2):17. doi: 10.3892/ijmm.2023.5220. PMID: 31257467 Free PMC article. Retracted.
-
Study on the mechanism of Shenkang injection in the treatment of chronic renal failure based on the strategy of "Network pharmacology-Molecular docking-Key target validation".PLoS One. 2023 Oct 5;18(10):e0291621. doi: 10.1371/journal.pone.0291621. eCollection 2023. PLoS One. 2023. PMID: 37796994 Free PMC article.
References
-
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941‐1952. - PubMed
-
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431‐440. - PubMed
-
- Cottin V. Idiopathic interstitial pneumonias with connective tissue diseases features: a review. Respirology. 2016;21:245‐258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

