Superhydrophobic Photosensitizers: Airborne 1O2 Killing of an in Vitro Oral Biofilm at the Plastron Interface
- PMID: 29972022
- PMCID: PMC6698391
- DOI: 10.1021/acsami.8b09439
Superhydrophobic Photosensitizers: Airborne 1O2 Killing of an in Vitro Oral Biofilm at the Plastron Interface
Abstract
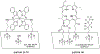
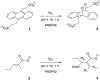
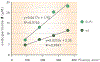
Singlet oxygen is a potent agent for the selective killing of a wide range of harmful cells; however, current delivery methods pose significant obstacles to its widespread use as a treatment agent. Limitations include the need for photosensitizer proximity to tissue because of the short (3.5 μs) lifetime of singlet oxygen in contact with water; the strong optical absorption of the photosensitizer, which limits the penetration depth; and hypoxic environments that restrict the concentration of available oxygen. In this article, we describe a novel superhydrophobic singlet oxygen delivery device for the selective inactivation of bacterial biofilms. The device addresses the current limitations by: immobilizing photosensitizer molecules onto inert silica particles; embedding the photosensitizer-containing particles into the plastron (i.e. the fluid-free space within a superhydrophobic surface between the solid substrate and fluid layer); distributing the particles along an optically transparent substrate such that they can be uniformly illuminated; enabling the penetration of oxygen via the contiguous vapor space defined by the plastron; and stabilizing the superhydrophobic state while avoiding the direct contact of the sensitizer to biomaterials. In this way, singlet oxygen generated on the sensitizer-containing particles can diffuse across the plastron and kill bacteria even deep within the hypoxic periodontal pockets. For the first time, we demonstrate complete biofilm inactivation (>5 log killing) of Porphyromonas gingivalis, a bacterium implicated in periodontal disease using the superhydrophobic singlet oxygen delivery device. The biofilms were cultured on hydroxyapatite disks and exposed to active and control surfaces to assess the killing efficiency as monitored by colony counting and confocal microscopy. Two sensitizer particle types, a silicon phthalocyanine sol-gel and a chlorin e6 derivative covalently bound to fluorinated silica, were evaluated; the biofilm killing efficiency was found to correlate with the amount of singlet oxygen detected in separate trapping studies. Finally, we discuss the applications of such devices in the treatment of periodontitis.
Keywords: biofilm eradication; dentistry; photodynamic therapy; singlet oxygen; superhydrophobic device.
Conflict of interest statement
The authors declare the following competing financial interest(s): Alexander Greer is co-founder and CTO and Alan Lyons is co-founder and COO of SingletO2 Therapeutics LLC.
Figures
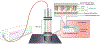




Similar articles
-
Antimicrobial Photodynamic Inactivation Using Topical and Superhydrophobic Sensitizer Techniques: A Perspective from Diffusion in Biofilms.Photochem Photobiol. 2021 Nov;97(6):1266-1277. doi: 10.1111/php.13461. Epub 2021 Jul 8. Photochem Photobiol. 2021. PMID: 34097752 Free PMC article. Review.
-
Evaluation of photosensitizer-containing superhydrophobic surfaces for the antibacterial treatment of periodontal biofilms.J Photochem Photobiol B. 2022 Aug;233:112458. doi: 10.1016/j.jphotobiol.2022.112458. Epub 2022 Apr 29. J Photochem Photobiol B. 2022. PMID: 35691161 Free PMC article.
-
Singlet oxygen generation on porous superhydrophobic surfaces: effect of gas flow and sensitizer wetting on trapping efficiency.J Phys Chem A. 2014 Nov 13;118(45):10364-71. doi: 10.1021/jp503149x. Epub 2014 Jun 13. J Phys Chem A. 2014. PMID: 24885074 Free PMC article.
-
Superhydrophobic photosensitizers. Mechanistic studies of (1)O2 generation in the plastron and solid/liquid droplet interface.J Am Chem Soc. 2013 Dec 18;135(50):18990-8. doi: 10.1021/ja410529q. Epub 2013 Dec 10. J Am Chem Soc. 2013. PMID: 24295210 Free PMC article.
-
Photodynamic therapy in dentistry.J Dent Res. 2007 Aug;86(8):694-707. doi: 10.1177/154405910708600803. J Dent Res. 2007. PMID: 17652195 Review.
Cited by
-
Singlet oxygen generation on a superhydrophobic surface: Effect of photosensitizer coating and incident wavelength on 1O2 yields.Photochem Photobiol. 2025 Jan-Feb;101(1):167-179. doi: 10.1111/php.13969. Epub 2024 Jun 2. Photochem Photobiol. 2025. PMID: 38824412
-
Light-activated antimicrobial coatings: the great potential of organic photosensitizers.RSC Adv. 2025 Mar 13;15(10):7905-7925. doi: 10.1039/d5ra00272a. eCollection 2025 Mar 6. RSC Adv. 2025. PMID: 40084300 Free PMC article. Review.
-
Subcritical Water-Driven Complete Mineralization of Silane Coupling Agents with Short Perfluoroalkyl Chains.ACS Omega. 2025 Jul 31;10(31):35086-35094. doi: 10.1021/acsomega.5c04738. eCollection 2025 Aug 12. ACS Omega. 2025. PMID: 40821544 Free PMC article.
-
Antimicrobial Photodynamic Inactivation Using Topical and Superhydrophobic Sensitizer Techniques: A Perspective from Diffusion in Biofilms.Photochem Photobiol. 2021 Nov;97(6):1266-1277. doi: 10.1111/php.13461. Epub 2021 Jul 8. Photochem Photobiol. 2021. PMID: 34097752 Free PMC article. Review.
-
pH responsive superporogen combined with PDT based on poly Ce6 ionic liquid grafted on SiO2 for combating MRSA biofilm infection.Theranostics. 2020 Mar 26;10(11):4795-4808. doi: 10.7150/thno.42922. eCollection 2020. Theranostics. 2020. PMID: 32308750 Free PMC article.
References
-
- Singlet Oxygen: Applications in Biosciences and Nanosciences. Nonell S, Flors C, Eds, Royal Society of Chemistry, Oxfordshire, UK: 2016, pp. 1–450.
-
- Pryor WA; Houk KN; Foote CS; Fukuto JM; Ignarro LJ; Squadrito GL; Davies KJ Free Radical Biology and Medicine: It’s a Gas, Man! Am. J. Physiol. Regul. Integr. Comp. Physiol 2006, 291, R491–R511. - PubMed
-
- Krinsky NI Biological Roles of Singlet Oxygen In: Wasserman HH, Ed. Singlet Oxygen Vol. 40 Academic Press, 1979, pp. 597–641.
-
- Felgenträger A; Maisch T; Späth A; Schröder JA; Bäumler W Singlet Oxygen Generation in Porphyrin-Doped Polymeric Surface Coating Enables Antimicrobial Effects on Staphylococcus Aureus. Phys. Chem. Chem. Phys 2014, 16, 20598–20607. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

