1α,25-dihydroxyvitamin D3-eluting nanofibrous dressings induce endogenous antimicrobial peptide expression
- PMID: 29972648
- PMCID: PMC6219435
- DOI: 10.2217/nnm-2018-0011
1α,25-dihydroxyvitamin D3-eluting nanofibrous dressings induce endogenous antimicrobial peptide expression
Abstract
Aim: The aim of this study was to develop a nanofiber-based dressing capable of local sustained delivery of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) and augmenting human CAMP induction.
Materials & methods: Nanofibrous wound dressings containing 1,25(OH)2D3 were successfully prepared by electrospinning, which were examined in vitro, in vivo and ex vivo.
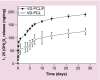
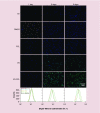
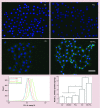
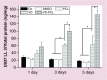
Results: 1,25(OH)2D3 was successfully loaded into nanofibers with encapsulation efficiency larger than 90%. 1,25(OH)2D3 showed a sustained release from nanofibers over 4 weeks. Treatment of U937 and HaCaT cells with 1,25(OH)2D3-loaded poly(ϵ-caprolactone) nanofibers significantly induced hCAP18/LL37 expression in monocytes and keratinocytes, skin wounds of humanized transgenic mice and artificial wounds of human skin explants.
Conclusion: 1,25(OH)2D3 containing nanofibrous dressings could enhance innate immunity by inducing antimicrobial peptide production.
Keywords: 1α,25-dihydroxyvitamin D3; antimicrobial peptides LL-37; electrospun nanofibers; endogenous production; innate immunity; sustained delivery.
Conflict of interest statement
This work was supported by grants from the National Institute of General Medical Science (NIGMS) at the NIH (2P20 GM103480-06 and 1R01GM123081 to J Xie), the Otis Glebe Medical Research Foundation and startup funds from the University of Nebraska Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations orfinancial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures




References
-
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999;27(2):97–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
